26.03.2013
•
Views
d r- 0 r- ,-I d N 2 N 2 co Ln N 0 m m N m d d 0 d 4 m d N 4 0 m N d co m d h 2 d h N 4 E) d N 0 N QI m N 0 4 N m N 0 d .s 0% N e .. c a d 00 co 4 U 4 a c 0 U W * ?N.g W *
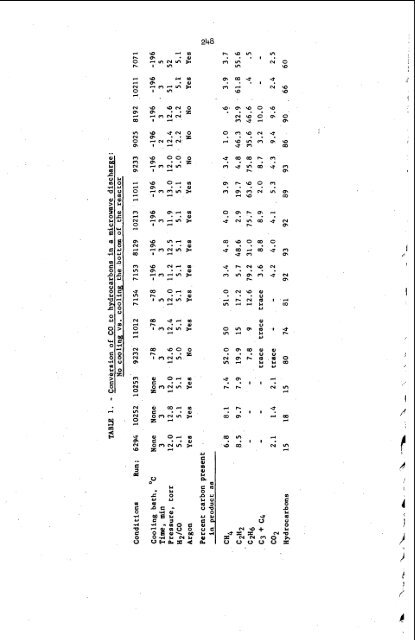
The next five coluws in table 1 show that with liquid N2 cooling (-196'), the percent of carbon in the product as CH4 in only 4%; C2H2 + C2H6 (with the latter predominating) 81%; C3 + C4 hydrocarbons, 69.; more H20 and C02 are also formed. The mass spectrometric analyses indicated that traces of oxygenates were also present in the products of these runs, but in such small amunts that no identi- fications could be attempted. For some unknown reason, C2H4 is not formed under these conditions. (Several runs were analyzed by gas chromatography For C2H4 and none was found.) At a temperature of -196O, the only non-condensables present in the mixture are H2, CO, and CH4; all the other products are frozen but. One can speculate that the high yield of C2H6 is due either to a recombination of CH3 radicals at the cold surface, or hydrogenation of C2H2, but the mechanism of formation of any of the hydrocarbon products is not known. The next-to-last pair of columns in table 1 show that very high conversions of CO to C2- and higher hydrocarbons can be obtained using a 2.2:l H + CO mixture. Here, also, the C02 production is higher, and the CH4 lower, tian compared with the more hydrogen-rich reactant mixture. The last pair of columns, giving data for runs made at 50 torr, indicate that C2H2 was, by far, the predominant product in these runs. The almost complete conversion of CO to hydrocarbonqH20, and C02, obtained by cooling the bottom of the reactor, is reversible. Several additional experiments (at 12 torr), where the gases were reacted for 3 minutes while the reactor was cooled, the bottom of the reactor then warmed to room temperature in a few seconds with a water bath, and the gases reacted for 2 more minutes, gave the same product compositions as for the runs shown in figures 1 and 2. Table 2 gives the results of some preliminary experiments where water vapor was added to the H2 + CO + A r mixture before reaction. The water vapor was added to the previously evacuated reactor and a portion of the vacuum system connected to a Pace Engineering Co. Model P7 pressure transducer containing a + 1 psi diaphragm. The output of this was indicated on a Pace Model CD25 transducer indicator. After the water vapor partial pressure was measured, the stopcock to the reactor was closed and the water frozen at,the bottom of the reactor. The reactor was then filled with the H2-CO-Ar mixture, the bottom of the reactor warmed slightly, and 3 minutes allowed for mixing of the gases before the discharge was initiated. Even from these few experiments, it can be seen that adding water vapor to the initial reactant mixture has a strong inhibitory effect on the production of hydrocarbons. Comparison of the second and third runs listed in the table shows that 3 minutes reaction time increases the yield of hydrocarbons only slightly as compared to'one minute. The last two runs in the table show that when the water vapor partial pressure is equal to or greater than the CO partial pressure in the reactant gas mixture, no hydrocarbons are produced at all. (The C02 yield is increased due to the reaction H20 + CO +C02 + H2.) The pronounced inhibitory effect of H20 vapor on hydrocarbon formation in this reaction may explain some of the scatter of the data shown in figures 1, 2, and 3. It is possible that small (and variable) amounts of H20 vapor were adsorbed on the walls of the reactor tubes, and that this decreased the hydrocarbon yield in (some of) the runs by a varying amount which shows up as scatter in the data. Further experiments would have to be done to shed more light on this point. However, there are other, as yet unknown, sources of variability in the experimental conditions which also undoubtedly contribute to the scatter in the data.
-
Page 1 and 2:
)i 1 reesonably complete and accura
-
Page 3 and 4:
3 inquire about the component of ve
-
Page 5 and 6:
4 I I 5 To find (t2)av, we assume t
-
Page 7 and 8:
\ vnich, it is noted can be negativ
-
Page 9 and 10:
I 7 than or smaller than the second
-
Page 11 and 12:
i I i I I I ) ) i L , I I . INT1:OD
-
Page 13 and 14:
13 field per electron is and per co
-
Page 15 and 16:
. 11. 5. CharRe-Transfer and Ion-Mo
-
Page 17 and 18:
17 In the followin:: sections much
-
Page 19 and 20:
above. With that EIN, an estimate o
-
Page 21 and 22:
21 region in w!iich large, nighlv l
-
Page 23 and 24:
i ’ understanding: 23 (a) Electro
-
Page 25 and 26:
Obviously, the secondary ion must h
-
Page 27 and 28:
27 (22) Franklin, J. L., Munson, M.
-
Page 29 and 30:
Tables 1-6 present examples of rela
-
Page 31 and 32:
Table 8 Some Ions Formed by Process
-
Page 33 and 34:
33 velocity of the reacting partn r
-
Page 35 and 36:
35 must be added to the Langevin cr
-
Page 37 and 38:
37 In our laboratory a microwave di
-
Page 39 and 40:
+ . 4 . r 39 as well as N in a cor0
-
Page 41 and 42:
ION-MOIECULE REACTION RATES MEASUFI
-
Page 43 and 44:
43 excitntion 2onditions so that th
-
Page 45 and 46:
45 In 2 like manner Fig. 3, showing
-
Page 47:
47 Absorption Spectra of Transient
-
Page 50 and 51:
50 maximum fiela. In this manner, a
-
Page 52 and 53:
52 3 % %- %- 5- a- 7 h W P H 0 L v)
-
Page 54 and 55:
References I I . A.B.Callear, J.A.G
-
Page 56 and 57:
._ . _-.. - , , . . ,. . The Pyrex
-
Page 58 and 59:
58 0 0 0 VI J > I cv . u H a
-
Page 60:
.... . 0 2 e I / m 0 H F4 : 1
-
Page 63 and 64:
\ 0.9 0.8 0.7 06 0.5 0.4 0.3 0.2 01
-
Page 65 and 66:
65 ' cause of the reduction in the
-
Page 67 and 68:
INTRODUCTION I' Attachment of polar
-
Page 69 and 70:
I 69 EXPERIMENTAL The mass spectrom
-
Page 71 and 72:
+ Figure 1 Mixed water and methanol
-
Page 73 and 74:
73 ' , radius might be expected bec
-
Page 75 and 76:
75 Negative Ion Mass Spectra of Som
-
Page 77 and 78:
A b 1 1 I H I I I FILAMEN T CONTINU
-
Page 79 and 80:
79 for positive and negative ions,
-
Page 81 and 82:
51 out to answer some of the questi
-
Page 83 and 84:
93 INTERACTIONS OF EXCITED SPECIES
-
Page 85 and 86:
L 4 I*C z 0'2 = a, a U x I m 4 m 0
-
Page 87 and 88:
I L I, ; > > E Fig. 3 I50 200 150 1
-
Page 89 and 90:
I 300 r 1 - 200 i io ;' a cn I I. 0
-
Page 91 and 92:
where DISCUSSION OF XESULTS PERTAIN
-
Page 93 and 94:
93 where the bar indicates values c
-
Page 95 and 96:
'I i 'I 0 2. 4 6 8 2 [ N]* x' I 0-2
-
Page 97 and 98:
Fig. 14 IO 8 6 4F [NO] x IO-" (mole
-
Page 99 and 100:
for chemiluminescent excitation in
-
Page 101 and 102:
101 Chemiluminescent Reactions of E
-
Page 103 and 104:
103 All gases were taken directly f
-
Page 106 and 107:
10; He: + N2 -. 2He + h': (8) whi!?
-
Page 108 and 109:
description for both diffusion and
-
Page 110 and 111:
B. Ions and Electrons I Consider a
-
Page 112 and 113:
' 112 axial diffusion through the d
-
Page 114 and 115:
the tube walls occurs continuously
-
Page 116 and 117:
E2R M ' $6"
-
Page 118 and 119:
1. 2. 3. 4. 5. 6. 7. 8. 9- 10. u. -
-
Page 120 and 121:
120 . Dlschorge zone Reactor zone 1
-
Page 122 and 123:
122 In radiation chemistry the cust
-
Page 124 and 125:
124 4. chemistry seem limited essen
-
Page 126 and 127:
126 Conversion of Mixtures into Mor
-
Page 128 and 129:
Hydrazine Synthesis in A Silent dle
-
Page 130 and 131:
{;I . - . .. . - .., . . .. _- .. .
-
Page 132 and 133:
1 \ \ \ , \ , \ \ Pmer hnrity K.W.
-
Page 134 and 135:
. I operating fact that the sloGe i
-
Page 136 and 137:
_- of ' . 8. - 7 6, Residence Time
-
Page 138 and 139:
135 Ionic Reactions in Corona Disch
-
Page 140 and 141:
GAS OUT GAS IN i.ho QUADRUPOLE MASS
-
Page 142 and 143:
- + ( ~ ~ + 0 H ) ~ O ~ ( H~o)~H+ +
-
Page 144 and 145:
144 i I , , , , . + 1- u 3c w Inu c
-
Page 146 and 147:
146 SYNTHESIS OF ORGANIC COIGhTDS B
-
Page 148 and 149:
mi5 a- dz CI n I a I n +4 - 0 -I- k
-
Page 150 and 151:
0 z cu I I I
-
Page 152:
I- I - t d m a .rl Y x c, t-' d k
-
Page 155 and 156:
, 155 This result is significant in
-
Page 157 and 158:
Compound Bond he rgy Li I 82 UBr 10
-
Page 159 and 160:
, ”..’ 3or 25 - 5 20 - s 3 w Y
-
Page 161 and 162:
I 161 THE GLOW DISCHARGE DEPOSITION
-
Page 163 and 164:
Fig. 1 Process Apparatus -__l.ll__
-
Page 165 and 166:
v) t- at- InIo Io0 t-Q) loo lcua O
-
Page 167 and 168:
This study is only an approximation
-
Page 169 and 170:
Mole Reaction Material ratio time e
-
Page 171 and 172:
\ i (4) Effect of System Pressure T
-
Page 173 and 174:
173 Pressure. Since pressure is a c
-
Page 175 and 176:
175 Table VI X-RAY DATA OF DEPOSIT
-
Page 177 and 178:
177 Another source of weakness is t
-
Page 179 and 180:
179 PLATING IN A CORONA DISCHARGE R
-
Page 181 and 182:
\ 3 , ,\ 110 v 60 CPS MANOMETER . F
-
Page 183 and 184:
I i , \ I i & a - P Fig. 3 Reaction
-
Page 185 and 186:
\ \' C 0 .d * d .- C U d 0) c( 1) L
-
Page 187 and 188:
I i I . Q) I, s w 0 v) Y 0 a w W a
-
Page 189 and 190:
I (b) Polarized Light Fig. 6 Cross
-
Page 191 and 192:
i m v) Y C i! u o) 23 a a- + 191 m
-
Page 193 and 194:
i, d C .d c c W Q ) 0 0 L1Y 0 000 0
-
Page 195 and 196:
i e 4 0 wcr -4 4 al 0 0 0 c) cr 13:
-
Page 197 and 198:
\ E ‘ 1 TIME FOR RUN 13-1 14-1 -
-
Page 199 and 200:
199 a red heat in this cell using d
-
Page 201 and 202:
\ , t j \ I, v) 2 Y . C 0 u m .I C
-
Page 203 and 204:
203 VAPOR PHASE FORMATION OF NONCRY
-
Page 205 and 206:
BELL JAR STANC TO VACUUM SYSTEM U T
-
Page 207 and 208:
207 of the boron oxide film, the fi
-
Page 209 and 210:
i I, 4 I.( Y I- * 0 i f i 3 0.t I I
-
Page 211 and 212:
211 The Reacticn 3f Oxygen with Car
-
Page 213 and 214:
2 E, W t- a Z 9 I- a 2 X 0 I50 too
-
Page 215 and 216:
'i' 100 50 IC 21 5 2.0 2.5 SURFACE
-
Page 217 and 218:
I- z W 0 w a a 100 80 60 40 20 215
-
Page 219 and 220:
s., Literature Cited Berkley, C. ,
-
Page 221 and 222:
7 h \ F 221 600°C and 1 atmosphere
-
Page 223 and 224:
223 e I'
-
Page 225 and 226:
R L ' -1 9mm O.D. 5mm I.D. 45mm 0.D
-
Page 227 and 228:
Figure 5.- Paschen's law curves. 2-
-
Page 229 and 230:
\ '? I The water-free product gases
-
Page 231 and 232:
N I + 0" ON i I + 0 u / 0 0 I 0 cu
-
Page 233 and 234:
- 233 SOME PROBLEMS OF THE KINETICS
-
Page 235 and 236:
.. . :. ,. .I , . i ? .. . , ... .
-
Page 237 and 238:
237 Table 2 Experimental Variables
-
Page 239 and 240:
239 Zhbie I Eerccnt Consumption of
-
Page 241 and 242:
241 Discussion The systematic varia
-
Page 243 and 244:
I \ 1 243 FORMATION OF HYDROCARBONS
-
Page 245 and 246:
h z - a o m a 20 T IME, minutes . F
-
Page 247:
I 1 I I I I 0 I 2 3 4 5 TIME, minut
-
Page 251 and 252:
\ .\ t 251 Given the existence of t
-
Page 253 and 254:
I L 253 Epple, R. P. and C. M. Apt.
-
Page 255 and 256:
1 ' Fig. 1 Schematic of flow discha
-
Page 257 and 258:
5' . ' I 1 257 co, with excess H2,
-
Page 259 and 260:
1 1 1 moo 1400 1300 c V' I IPOO I I
-
Page 261 and 262:
i 25i The Dlssocfatior of ToIuere V
-
Page 263 and 264:
L ! , c PRODUCTS FROM A 28 MC. DISC
-
Page 265 and 266:
'\ J ?. , .. . : PRODUCT FORMATION
-
Page 267 and 268:
'9 / I '\ "1 BENZYL CATION AND RADI
-
Page 269 and 270:
a
-
Page 271 and 272:
271 tube serve? as the high voltare
-
Page 273 and 274:
273 ion (1L.). Zxcitei-ion by colli
-
Page 275 and 276:
Apparatus and Procedure 275 EXPERIM
-
Page 277 and 278:
. 277 6. A freshly prepared sam le
-
Page 279 and 280:
observed. The polystyrene (10% solu
-
Page 281 and 282:
i i (12) c (11) (14) I 281 LITERATU
-
Page 283 and 284:
FLOW- METER I ADDITIVE SUPPLY * ,28
-
Page 285 and 286:
285 TABLE 2 Effect of Haloqenated A
-
Page 287 and 288:
ANALYTICAL RESULTS Some evidence wa
-
Page 289 and 290:
DISCUSSION 289 The salient features
-
Page 291 and 292:
? ~ MENBERSHIP IN THE DIVISION OF F
-
Page 293 and 294:
292 flow of the reactant gas stream
-
Page 295 and 296:
294 have suitable residence times i
-
Page 297 and 298:
0 E < h( E l! d K c 0 .- Y 0 t u t
-
Page 299 and 300:
298 yields of many chemical product
-
Page 301 and 302:
- ;i Y . 15. Pressure lOmm 5 > 10-
-
Page 303 and 304:
302 the best and in many practical
-
Page 305 and 306:
GENERATION AND MEASUREMENT OF AUDIO
-
Page 307 and 308:
. The transformer secondary and the
-
Page 309 and 310:
Vaccum-Tube Amplifier The mobile co
-
Page 311 and 312:
310 f = Power supply frequency in c
-
Page 313:
FUFARCI! INSTITUTE OF TFYDLC UNIVER
-
Page 316 and 317:
J # I / 4) I . . '8 r4 0 r( P
-
Page 318 and 319:
317 i Cuencb svsten w.nnar;itus use
-
Page 320 and 321:
epcrte? the noss~kil.itv of nroduct
-
Page 322 and 323:
1' b t +
-
Page 324 and 325:
323 ....... - . . . . . . . . . . .
-
Page 326 and 327:
1 \ i I i , / / / 8 3 1 325 To205 +
-
Page 328 and 329:
- .- - - . - - . . . - . 327 n + c
-
Page 330 and 331:
' POWER PLASMA GAS l e , ' I ! I I
-
Page 332 and 333:
331 f'ENI?AL PF'FCLCCFC Dernis, P.R
-
Page 334 and 335:
i J 333 ; mosphere (as opposed to a
-
Page 336 and 337:
; it raised the question, still &?z
-
Page 338 and 339:
p .I V I .= - - HCN/C(CALC. WITH C
-
Page 340 and 341:
1 1 339 atmospheric ressure and ach
-
Page 342 and 343:
RESULTS 341 Composition Dependence
-
Page 344 and 345:
0.09 0.08 0.07 9 0.06 2 U - .$ 0.05
-
Page 346 and 347:
II \ to the Slot So that less of th
-
Page 348 and 349:
i 4 2 HYDROCARBON-NITROGEN REACTION
-
Page 350 and 351:
I 1 349 c M m ; a e, k M x
-
Page 352 and 353:
1.0 I - a. I n TEMPERATURE - OK Fig
-
Page 354 and 355:
353 the experimental results in the
-
Page 356 and 357:
,) 355 The ceaswed conversion cf ni
-
Page 358 and 359:
1 6 357 Freezing. 3jpotnesise thzf
-
Page 360 and 361:
359 \ Frozen Compositior: Calcillat
-
Page 362 and 363:
I 1 \ 361 -. ne reaction proceecis
-
Page 364 and 365:
\ 36 3 ' h, I 26. H. Purnell, Gas C
-
Page 366 and 367:
In experiments on the direct conver
-
Page 368 and 369:
367 used as blnders, with different
-
Page 370 and 371:
369 ' i 1. REFERENCES Berber, John
-
Page 372 and 373:
Distribution and yield rates of pro
-
Page 374 and 375:
. . I . I 370 . . . . ., . . .. . .
-
Page 376 and 377:
4 372 090- > E w - m c N O - 0 z :
-
Page 378 and 379:
A-Feed tank 6-Thermocracker GReceiv
-
Page 380 and 381:
80 \ 0 70 60 e 40 a U VI 9 30 20 10
-
Page 382 and 383:
COAL DEASH& AND HIGH-PURITY COKE Wa
-
Page 384 and 385:
3Pn _. -. - L -J:,J.-;~~~L, ):as se
-
Page 386 and 387:
S ~ l ~ o n~pg:ading r is the resul
-
Page 388 and 389:
The range ~f temperatures and gener
-
Page 390 and 391:
. .. 386 9 * - r. Y 2 .' d a f' r.
-
Page 392 and 393:
.) .L m 388 I
-
Page 394 and 395:
TABLE 4 390 DELAYED COKING OF DEASH
-
Page 396 and 397:
, ! I- on '---
-
Page 398 and 399:
394 The present method is to keep t
-
Page 400 and 401:
396 ‘c erperature, while the pres
-
Page 402 and 403:
2, '! ! Proximate Analysis, wt $ Mo
-
Page 404 and 405:
0 IS 0 14 LL 9 013 e \ 3 b- m *- 01
-
Page 406 and 407:
402 HYDROGEN CYANIDE PRODUCED FROM
-
Page 408 and 409:
404 Before startup, the system is p
-
Page 410 and 411:
4 OL. ' , I. . TesCs. With.Coals .o
-
Page 412 and 413:
Table 3.- Product Gas Analyses and
-
Page 414 and 415:
410 BIBLIOGRAPHY 1. American Public
-
Page 416 and 417:
Figure 2. Enclosure surrounding hyd
-
Page 418 and 419:
0 0 f v) C 0 u m I z 2 c 2 r d J w
-
Page 420 and 421:
0. E c .4 7 .. .c . I ( - Low tempe
-
Page 422 and 423:
418 cual. A mjor aa\;antage or' the
-
Page 424 and 425:
420 Ir ?Y ? ? 9 9 9 pc rod n o c o
-
Page 426 and 427:
422 Table 3. Room-texperature M'dss
-
Page 428 and 429:
424 state, depending on the strengt
-
Page 430 and 431:
Table 4. Room-?;ergerature isomer s
-
Page 432 and 433:
I' 1.3 . /o 9 IO 9 6 a 425 F F 33 I
-
Page 434 and 435:
Brooks J.D. and Sternhell S. (1957)
-
Page 436 and 437:
(52) Gib3 T.C. and Greenwood N.N. (
-
Page 438 and 439:
434 transducer vas then introduced
-
Page 440 and 441:
436 yield is quite similar. The con
-
Page 442 and 443:
?-!??? 0 mv, Uh\OhIe . . . . eirlrl
-
Page 444 and 445:
4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 4
-
Page 446 and 447:
442 CHEMICAL REACTIONS IN A CORONA
-
Page 448 and 449:
helium 444 CORONA REACTOR SYSTEM Fi
-
Page 450 and 451:
446 The ultraviolet spectrum. for t
-
Page 452 and 453:
Biphenyl Fraction 448 The low molec
-
Page 454 and 455:
LUd The actual structural definitio
-
Page 456 and 457:
I I I I I In m 9 In 2 , I: r- In m
-
Page 458 and 459:
’. 454 Mechanism I The available
-
Page 460 and 461:
456 , , . The relatively low yield
-
Page 462 and 463:
458 h/lethylacetylene, allene and b
-
Page 464 and 465:
Introduction VAPOR PHASE DECOMPOSIT
-
Page 466 and 467:
462 more efficient hydrogenation. T
-
Page 468 and 469:
464 place in parallel with fragment
-
Page 470 and 471:
466 P rl 0, k 7 rnI rn al k a I hl
-
Page 472 and 473:
458 TABLE I. COMPOSI'IION OF GASEOU
-
Page 474 and 475:
REFERENCES 1. C. Hirayama and D. A.
-
Page 476 and 477:
i 472 Reciprocal Arc Enthalpy ,-> F
-
Page 478 and 479:
474 be pumped down without altering
-
Page 480 and 481:
476 Instead a hydrogen deficient fi
-
Page 482 and 483:
i 478
-
Page 484 and 485:
480 FATTY ACIDS AND n-ALKANES IN GR
-
Page 486 and 487:
482 TA5L,E 2. - Carbon number distr
-
Page 488 and 489:
6. 7. a. 9. 484 Lawlor, D. L., and
-
Page 490 and 491:
' I ' Sample No. 6 ' 12 IO 8 6 z 4
-
Page 492 and 493:
. 488 uiolecular weight of which ca
-
Page 494 and 495:
Xississippi (Sample GS). Stock solu
-
Page 496 and 497:
492 found at 5.7 and 4.9& for the W
-
Page 498 and 499:
494 mechanisms. However, the voltad
-
Page 500 and 501:
For pure polynuclcar arom.i;ic hydr
-
Page 502 and 503:
- '-'. . ' . . values are listed in
-
Page 504 and 505:
500 (1;) Ten, T. 17.: a;1d,Dic;
-
Page 506 and 507:
- ~ Resistivity Czlculation 2: 1 j:
-
Page 508 and 509:
504 o ' C 0s 2 0 v x i 8 2 0 ! P -
-
Page 510 and 511:
506 i
-
Page 512 and 513:
d 601 L P LL
-
Page 516 and 517:
I I I I I I I I m W N W N 0 (D ? 0
-
Page 518 and 519:
514 was heated under nitrogen in a
-
Page 520 and 521:
The line broadening at 79OK of the
-
Page 522 and 523:
13. 14. 15. 16. 17. 18. 19. 20. 21.
-
Page 524 and 525:
W L, Z 6 m (1: 0 6 d j o 2 ' 1 0.0
-
Page 526 and 527:
Coals 522 Studies of the 1600 cm-l
-
Page 528 and 529:
L in ole i c a c id - 0' Two sample
-
Page 530 and 531:
526 Table 1.- Ultimate analyses of
-
Page 532 and 533:
528 6. Friedel, R. A., and H. Retco
-
Page 534 and 535:
egeneration. The system can be seen
-
Page 536 and 537:
532 and can be expressed in terms o
-
Page 538 and 539:
534 in the vapor increases with inc
-
Page 540 and 541:
NH3 NH3 NH3 NH3 Pyridine Pyridine P
-
Page 542 and 543:
Mole Ratio of Test No. Quinoline to
-
Page 544 and 545:
IXPROFJCTION D; M. Mason Institute
-
Page 546 and 547:
-4:c~rciiny to recent studies o ;i4
-
Page 548 and 549:
544 Table 1. LIGHT EMISSION OF Y"TR
-
Page 550 and 551:
emission in a few cases may be by d
-
Page 552 and 553:
LITERATUR2 CITED i. C. -. .' * il.
-
Page 554 and 555:
co, CH,,OR 1 - OTHER ADDITIVE FRITT
-
Page 556 and 557:
FRIT 'TED CO, CH,.OR 4 ___ OTHER AD
-
Page 558 and 559:
340 350 300 400 4 50 500 600 700 80
-
Page 560 and 561:
0 554 WITH COOLING (PLATE AT 40°C)
-
Page 562 and 563:
5 56 then t = to when e = 0 2) the
-
Page 564 and 565:
5 58 The surface heat transfer coef
-
Page 566 and 567:
Discussion and Results 560 Three br
-
Page 568 and 569:
__- ._ 562 Table I THERMAL AND PHYS
-
Page 570:
ln 4 I 0 4J v \ 1.0 0.8 0.6 L - 0.4