chemical physics of discharges - Argonne National Laboratory
chemical physics of discharges - Argonne National Laboratory chemical physics of discharges - Argonne National Laboratory
1 \ \ \ , \ , \ \ Pmer hnrity K.W. /ccxiD)
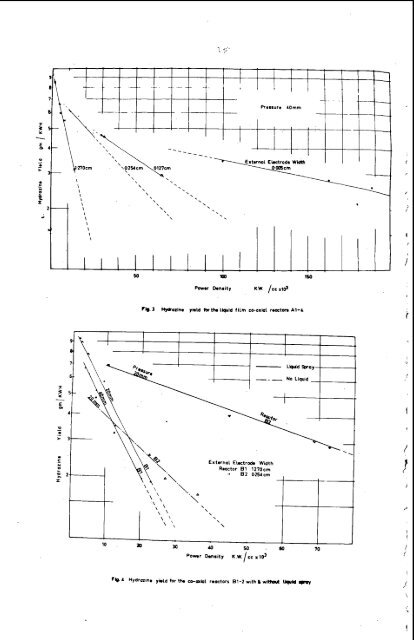
. a b c liqqid *iea.ctor L.!n/iLWH ~3a-l c c/Kwat t absorbent Al .l3.14 O.OUB7 73.81 firm 81 6.33 3.02j6 118.74 none A2 10.70 (O.OU87) 19.43 f iliil I1 I1 A3 9.56 12-93 11 A4 7.28 3.73 II B1 12.99 0*0098 73.58 spray 32 9.26 (0.0098) . 13.69 II B2 8.88 (U.0236) 35.15 none This is a reasoliable procedure within any one 1,erticular series of reactors such as A1 to 4, but it is OiQY Eiproximate witii iiiffering reactor series( e.g. Reactor series A and B)due to dissimilarities in discharge geometries. JJi,c;uSSI #If. The i?ost notable overall feature of the results is the .qrogresuive increase in hydrazine yield obteined by tite use of a liqu-id absorbent and by pihing tne discharge. The himest averase. jield was obtained wit!i tile pulsing technique with or without liqcdd absorbent aid was approxinietely 15 gms if H /KMH. Isolated values as nigi? as 20 @as/ W h were obtaine8 %ut further experimental work is required before these values can be made reproducible. It is noteworthy that a yield of 15 pis/KMH is beginning to look comercially attractive although it must be remenbered that the fiiL;,.l. product cost will depend to a great extent on the cost of the product purification process downstream of the . reactor. There are several interesting features shown by this work which bear discussion. From the results set out in Fig. 2 to 4 it is evident that the hydrazine yield decreases with increasing discharge power intensity despite a corresponding increase in the decomposition of ammonia. The only reasonable explanation for this which 118s been advanced is that hydrazine is formed in the discharge bj a complex reaction mechanism and is subsequently decomposed by electron bombardment or other collision phenomena (4,6 ) . The use of a liquid absorbent resdts in a decrease in the slope of the energy yield-power density plot. This is well illustrated in both Pig. 2 and Fig.4 by comparing the slope of the plots with and drithout the use of liquid absorbent. This indicates that hydrazine is being reinoved from the diecharge by the liquid absorbent instead of being degraded. If the hydrazine were completely removed so that degradation did not occur the elope of this plot would be wither independent of discharge power or possibly positive. The very
- Page 81 and 82: 51 out to answer some of the questi
- Page 83 and 84: 93 INTERACTIONS OF EXCITED SPECIES
- Page 85 and 86: L 4 I*C z 0'2 = a, a U x I m 4 m 0
- Page 87 and 88: I L I, ; > > E Fig. 3 I50 200 150 1
- Page 89 and 90: I 300 r 1 - 200 i io ;' a cn I I. 0
- Page 91 and 92: where DISCUSSION OF XESULTS PERTAIN
- Page 93 and 94: 93 where the bar indicates values c
- Page 95 and 96: 'I i 'I 0 2. 4 6 8 2 [ N]* x' I 0-2
- Page 97 and 98: Fig. 14 IO 8 6 4F [NO] x IO-" (mole
- Page 99 and 100: for chemiluminescent excitation in
- Page 101 and 102: 101 Chemiluminescent Reactions of E
- Page 103 and 104: 103 All gases were taken directly f
- Page 106 and 107: 10; He: + N2 -. 2He + h': (8) whi!?
- Page 108 and 109: description for both diffusion and
- Page 110 and 111: B. Ions and Electrons I Consider a
- Page 112 and 113: ' 112 axial diffusion through the d
- Page 114 and 115: the tube walls occurs continuously
- Page 116 and 117: E2R M ' $6"
- Page 118 and 119: 1. 2. 3. 4. 5. 6. 7. 8. 9- 10. u. -
- Page 120 and 121: 120 . Dlschorge zone Reactor zone 1
- Page 122 and 123: 122 In radiation chemistry the cust
- Page 124 and 125: 124 4. chemistry seem limited essen
- Page 126 and 127: 126 Conversion of Mixtures into Mor
- Page 128 and 129: Hydrazine Synthesis in A Silent dle
- Page 130 and 131: {;I . - . .. . - .., . . .. _- .. .
- Page 134 and 135: . I operating fact that the sloGe i
- Page 136 and 137: _- of ' . 8. - 7 6, Residence Time
- Page 138 and 139: 135 Ionic Reactions in Corona Disch
- Page 140 and 141: GAS OUT GAS IN i.ho QUADRUPOLE MASS
- Page 142 and 143: - + ( ~ ~ + 0 H ) ~ O ~ ( H~o)~H+ +
- Page 144 and 145: 144 i I , , , , . + 1- u 3c w Inu c
- Page 146 and 147: 146 SYNTHESIS OF ORGANIC COIGhTDS B
- Page 148 and 149: mi5 a- dz CI n I a I n +4 - 0 -I- k
- Page 150 and 151: 0 z cu I I I
- Page 152: I- I - t d m a .rl Y x c, t-' d k
- Page 155 and 156: , 155 This result is significant in
- Page 157 and 158: Compound Bond he rgy Li I 82 UBr 10
- Page 159 and 160: , ”..’ 3or 25 - 5 20 - s 3 w Y
- Page 161 and 162: I 161 THE GLOW DISCHARGE DEPOSITION
- Page 163 and 164: Fig. 1 Process Apparatus -__l.ll__
- Page 165 and 166: v) t- at- InIo Io0 t-Q) loo lcua O
- Page 167 and 168: This study is only an approximation
- Page 169 and 170: Mole Reaction Material ratio time e
- Page 171 and 172: \ i (4) Effect of System Pressure T
- Page 173 and 174: 173 Pressure. Since pressure is a c
- Page 175 and 176: 175 Table VI X-RAY DATA OF DEPOSIT
- Page 177 and 178: 177 Another source of weakness is t
- Page 179 and 180: 179 PLATING IN A CORONA DISCHARGE R
- Page 181 and 182: \ 3 , ,\ 110 v 60 CPS MANOMETER . F
1<br />
\<br />
\<br />
\<br />
,<br />
\ ,<br />
\<br />
\<br />
Pmer hnrity K.W. /ccxiD)