Hyperbaric Oxygen Therapy - Hyperbaric Chamber Information ...
Hyperbaric Oxygen Therapy - Hyperbaric Chamber Information ...
Hyperbaric Oxygen Therapy - Hyperbaric Chamber Information ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
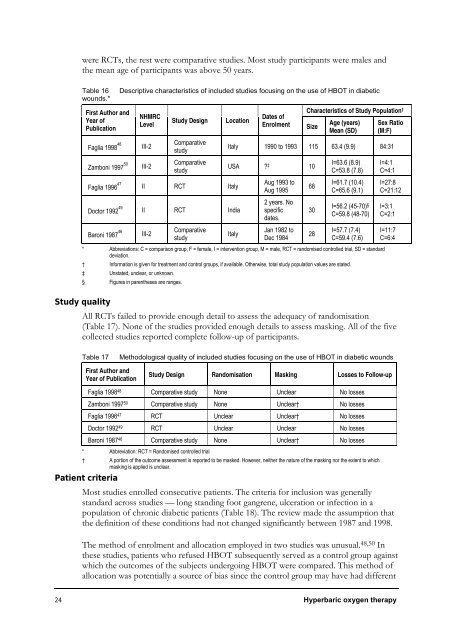
were RCTs, the rest were comparative studies. Most study participants were males and<br />
the mean age of participants was above 50 years.<br />
Table 16 Descriptive characteristics of included studies focusing on the use of HBOT in diabetic<br />
wounds.*<br />
First Author and<br />
Year of<br />
Publication<br />
Faglia 1998 48<br />
Zamboni 1997 50<br />
Faglia 1996 47<br />
Doctor 1992 49<br />
Baroni 1987 46<br />
NHMRC<br />
Level<br />
III-2<br />
III-2<br />
Study Design Location<br />
Comparative<br />
study<br />
Comparative<br />
study<br />
II RCT Italy<br />
II RCT India<br />
III-2<br />
Comparative<br />
study<br />
Dates of<br />
Enrolment Size<br />
Characteristics of Study Population †<br />
Age (years)<br />
Mean (SD)<br />
Sex Ratio<br />
(M:F)<br />
Italy 1990 to 1993 115 63.4 (9.9) 84:31<br />
USA ? ‡ 10<br />
Aug 1993 to<br />
Aug 1995<br />
2 years. No<br />
specific<br />
dates.<br />
I=63.6 (8.9)<br />
C=53.8 (7.8)<br />
I=4:1<br />
C=4:1<br />
24 <strong>Hyperbaric</strong> oxygen therapy<br />
Italy<br />
Jan 1982 to<br />
Dec 1984<br />
68<br />
30<br />
28<br />
I=61.7 (10.4)<br />
C=65.6 (9.1)<br />
I=56.2 (45-70) §<br />
C=59.8 (48-70)<br />
I=57.7 (7.4)<br />
C=59.4 (7.6)<br />
* Abbreviations: C = comparison group, F = female, I = intervention group, M = male, RCT = randomised controlled trial, SD = standard<br />
deviation.<br />
† <strong>Information</strong> is given for treatment and control groups, if available. Otherwise, total study population values are stated.<br />
‡ Unstated, unclear, or unknown.<br />
§ Figures in parentheses are ranges.<br />
I=27:8<br />
C=21:12<br />
I=3:1<br />
C=2:1<br />
I=11:7<br />
C=6:4<br />
Study quality<br />
All RCTs failed to provide enough detail to assess the adequacy of randomisation<br />
(Table 17). None of the studies provided enough details to assess masking. All of the five<br />
collected studies reported complete follow-up of participants.<br />
Table 17 Methodological quality of included studies focusing on the use of HBOT in diabetic wounds<br />
First Author and<br />
Year of Publication<br />
Study Design Randomisation Masking Losses to Follow-up<br />
Faglia 1998 48 Comparative study None Unclear No losses<br />
Zamboni 1997 50 Comparative study None Unclear† No losses<br />
Faglia 1996 47 RCT Unclear Unclear† No losses<br />
Doctor 1992 49 RCT Unclear Unclear No losses<br />
Baroni 1987 46 Comparative study None Unclear† No losses<br />
* Abbreviation: RCT = Randomised controlled trial<br />
† A portion of the outcome assessment is reported to be masked. However, neither the nature of the masking nor the extent to which<br />
masking is applied is unclear.<br />
Patient criteria<br />
Most studies enrolled consecutive patients. The criteria for inclusion was generally<br />
standard across studies — long standing foot gangrene, ulceration or infection in a<br />
population of chronic diabetic patients (Table 18). The review made the assumption that<br />
the definition of these conditions had not changed significantly between 1987 and 1998.<br />
The method of enrolment and allocation employed in two studies was unusual. 48,50 In<br />
these studies, patients who refused HBOT subsequently served as a control group against<br />
which the outcomes of the subjects undergoing HBOT were compared. This method of<br />
allocation was potentially a source of bias since the control group may have had different