C. HOW DOES YOUR ENVIRO BATTERY WORK? D ... - 4M-IND.com
C. HOW DOES YOUR ENVIRO BATTERY WORK? D ... - 4M-IND.com
C. HOW DOES YOUR ENVIRO BATTERY WORK? D ... - 4M-IND.com
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
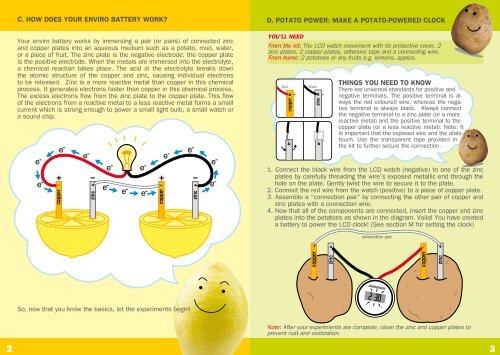
C. <strong>HOW</strong> <strong>DOES</strong> <strong>YOUR</strong> <strong>ENVIRO</strong> <strong>BATTERY</strong> <strong>WORK</strong>?<br />
Your enviro battery works by immersing a pair (or pairs) of connected zinc<br />
and copper plates into an aqueous medium such as a potato, mud, water,<br />
or a piece of fruit. The zinc plate is the negative electrode; the copper plate<br />
is the positive electrode. When the metals are immersed into the electrolyte,<br />
a chemical reaction takes place. The acid in the electrolyte breaks down<br />
the atomic structure of the copper and zinc, causing individual electrons<br />
to be released. Zinc is a more reactive metal than copper in this chemical<br />
process. It generates electrons faster than copper in this chemical process.<br />
The excess electrons flow from the zinc plate to the copper plate. This flow<br />
of the electrons from a reactive metal to a less reactive metal forms a small<br />
current which is strong enough to power a small light bulb, a small watch or<br />
a sound chip.<br />
copper<br />
zinc<br />
So, now that you know the basics, let the experiments begin!<br />
copper<br />
zinc<br />
D. POTATO POWER: MAKE A POTATO-POWERED CLOCK<br />
YOU’LL NEED<br />
From the kit: The LCD watch movement with its protective cover, 2<br />
zinc plates, 2 copper plates, adhesive tape and a connecting wire.<br />
Red Red Black Black<br />
From home: 2 potatoes or any fruits e.g. lemons, apples.<br />
copper<br />
copper<br />
Red Red Black Black<br />
copper<br />
copper<br />
zinc<br />
copper<br />
zinc<br />
zinc<br />
zinc<br />
zinc<br />
THINGS YOU NEED TO KNOW<br />
There are universal standards for positive and<br />
negative terminals. The positive terminal is always<br />
the red coloured wire, whereas the negative<br />
terminal is always black. Always connect<br />
the negative terminal to a zinc plate (or a more<br />
reactive metal) and the positive terminal to the<br />
copper plate (or a less reactive metal). Note: It<br />
is important that the exposed wire and the plate<br />
touch. Use the transparent tape provided in<br />
the kit to further secure the connection.<br />
1. Connect the black wire from the LCD watch (negative) to one of the zinc<br />
plates by carefully threading the wire’s exposed metallic end through the<br />
hole on the plate. Gently twist the wire to secure it to the plate.<br />
2. Connect the red wire from the watch (positive) to a piece of copper plate.<br />
3. Assemble a “connection pair” by connecting the other pair of copper and<br />
zinc plates with a connection wire.<br />
4. Now that all of the <strong>com</strong>ponents are connected, insert the copper and zinc<br />
plates into the potatoes as shown in the diagram. Voila! You have created<br />
a battery to power the LCD clock! (See section M for setting the clock)<br />
connection pair<br />
Note: After your experiments are <strong>com</strong>plete, clean the zinc and copper plates to<br />
prevent rust and oxidization.<br />
copper<br />
zinc
E. MUSICAL MUD: MAKE A SOUND - CHIP SING<br />
YOU’LL NEED<br />
From the kit: The electronic sound chip, 2 pairs of copper and zinc<br />
plates, adhesive tape, a connecting wire and a paper cup.<br />
From home: 2 small potted plants or 2 cups of garden dirt.<br />
1. Make sure the pots or cups of soil are reasonably moist.<br />
2. Connect the sound chip to a pair of copper and zinc plates using the same<br />
technique as in the mini clock (i.e. red wire to copper plate, black wire to<br />
zinc plate).<br />
3. Make a connection pair with the other zinc and copper plate as in D3.<br />
4. Insert the zinc and copper plates into to the soil as shown in the diagram.<br />
add some water<br />
copper<br />
zinc<br />
Did the chip sing?<br />
If the experiment worked, you should hear a faint noise <strong>com</strong>ing from the<br />
round metal plate of the sound chip. To amplify the sound,<br />
tape the base of the sound chip to the paper cup. The<br />
paper cup<br />
sound should now be louder. You should be able to<br />
hear a bird singing. Why? The paper cup resonates<br />
with the sound wave generated by the sound chip<br />
making it louder. Experiment using different<br />
“amplifiers” e.g. a water glass, a soda can etc. You’ll<br />
be amazed with the different sound effects they<br />
produce!<br />
copper<br />
zinc<br />
F. WATER WONDER: MAKE A <strong>BATTERY</strong> WITH WATER<br />
YOU’LL NEED<br />
From the kit: The light tower with LED lamp installed, 3 specially designed screw<br />
caps, 3 zinc plates, 3 copper plates and connecting wires. From home: Three<br />
small plastic water bottles (or you could simply use the cups provided, in this<br />
case, the specially designed screw cap is not needed).<br />
1. Fill three bottles with water.<br />
2. Connect the LED light on the light tower to a pair of zinc and copper plates<br />
as done in the previous experiments.<br />
3. Make 2 connection pair with the other zinc and copper plates.<br />
4. Insert the zinc and copper plates into the water containers as shown in the<br />
diagram. Make sure the plates do not touch each other as this will cause<br />
a short circuit and the LED lamp will not light up.<br />
Did the LED light up? Was it bright? Try adding some vinegar into the solution.<br />
Does this make the LED brighter? Can you explain why adding vinegar<br />
to the water would make a difference? Because water is neutral and metals<br />
are more reactive in acidic solutions, the current produced is stronger when<br />
vinegar, or other acidic solution, is added to the water. Now try using a salt<br />
solution, water and fruit juice. Record your findings on the experiment sheet.<br />
Which solution produces the best results and causes the LED to shine brightest?<br />
4 5<br />
copper<br />
zinc<br />
zinc<br />
copper<br />
copper<br />
zinc<br />
Remark: If the bottles used<br />
in this experiment are too<br />
tall, you may need to stand<br />
the light tower on the screw<br />
cap due to the connection<br />
wire’s length limit. On the<br />
other hand, you may choose<br />
to change to other display<br />
devices like the sound chip<br />
or LCD clock so that it could<br />
dangle naturally after the<br />
circuit is set.
copper<br />
copper<br />
aluminum foil<br />
soaked cotton<br />
coin<br />
aluminum foil<br />
soaked cotton<br />
coin
8<br />
I. FREAKY FORK: MAKE A FORK <strong>BATTERY</strong><br />
YOU’LL NEED<br />
From the kit: 2 zinc plates, LCD clock, adhesive tape and<br />
connection wire. From home: 2 forks, 1 lemon - halved.<br />
1. Connect one end of the red wire to the fork. Use a clothes peg<br />
or adhesive tape to secure the connection.<br />
2. Connect the black wire to the zinc plate.<br />
3. Now get another fork and zinc plate, connect them with a wire to<br />
make a “connection pair”.<br />
4. To activate the clock, insert all metals into the lemon as shown in the<br />
diagram.<br />
zinc<br />
How does it work?<br />
The fork acts like the positive electrode of the battery, like the copper plates<br />
in previous experiments. Most tableware utensils are plated with a metal<br />
which is less reactive than zinc. When both the utensils and zinc plates are<br />
inserted in the lemon, a reaction takes place. Electrons move from the zinc<br />
plates to the fork forming a current.<br />
J. ADDITIONAL EXPERIMENTS<br />
You can do more experiments by <strong>com</strong>bining those provided in the kit with<br />
materials from home. Here are some of the materials you could try:<br />
Electrolyte: Soda drink, salt water, fruit juice, different fruit etc. Positive Electrode:<br />
any kind of copper, copper plated metal and alloy, copper screw/nuts,<br />
copper key, copper foil, different kinds of brownish coins, copper wire, spoon.<br />
Negative Electrode: iron, aluminum, any kind of zinc plated metal, <strong>com</strong>mon<br />
screw/nuts/washer/nail, iron wire. Mix and match the different metals, the<br />
electrolytes and display devices. Record each of your findings on the record<br />
sheet and <strong>com</strong>pare the results. It’s fun to analyze your results and develop<br />
hypothesis for additional experiments.<br />
zinc<br />
K. EXPERIMENT RECORD SHEET<br />
*1 - 6 are experiments from section D - J.<br />
Positive<br />
Electrode<br />
Negative<br />
Electrode<br />
Electrolyte<br />
Display<br />
Media<br />
1* Copper Plate Zinc Plate Potato LCD Clock 2<br />
2*<br />
3*<br />
4*<br />
5*<br />
6*<br />
7<br />
8<br />
9<br />
10<br />
11<br />
12<br />
13<br />
14<br />
15<br />
16<br />
17<br />
18<br />
19<br />
20<br />
Copper Plate Zinc Plate Mud Sound Chip 2<br />
Copper Plate Zinc Plate Water (Vinegar) LED Lamp 2<br />
Copper Plate Paper Clip Soda drink Sound Chip 2<br />
Coin Aluminum Foil Vinegar Sound Chip 2<br />
Fork Zinc Plate Lemon LCD Clock 2<br />
Number of<br />
connections<br />
Comment<br />
9
L. FUN FACTS<br />
“Voltaic Pile” - Did you know that one of the first batteries was actually a stack<br />
of metal discs separated by cotton that was soaked in salt water? The coin<br />
experiment outlined in this kit is very similar. Although you used vinegar (it’s<br />
more acidic) instead of saltwater, the principle is exactly the same!<br />
Gaston Plante invented the first lead-acid battery in 1859, and Thomas Edison<br />
invented the first alkaline cell in 1914, less than 100 years ago! Can<br />
you imagine life without batteries? No flashlights, no CD or MP3 players, no<br />
handheld games, or digital watches! But that’s only the tip of the iceberg,<br />
there would be no hearing aids or digital thermometers, no remote toys, no<br />
cell phones, most calculators wouldn’t work, and there would be a handcrank<br />
on your parent’s car! What other items can you think of that require<br />
batteries?<br />
How are batteries recharged? Recharging a battery simply requires that you reverse<br />
the flow of electrons using a separate energy source such as electricity<br />
or solar panels. When the process is <strong>com</strong>plete, the positive and negative<br />
elements of the battery are restored to their original state and can be used<br />
again. The problem with recharging; however, is that the battery starts to<br />
loose its charge a little faster each time it is recharged. Scientists are looking<br />
for new types of batteries that don’t harm the environment and that can be<br />
replenished without using electricity.<br />
Why are store-bought batteries hazardous to the environment? Stop and think<br />
about it. Do you have any ideas? Well if you guessed that they are pollutants,<br />
you’re absolutely correct! The chemicals used in batteries eventually corrode<br />
through the battery casing and leak into the soil, eventually making its way<br />
into our water sources. Some of these chemicals, such as mercury, were<br />
considered so dangerous to the environment that they have been outlawed in<br />
certain countries! One of the most popular <strong>com</strong>ponents in today’s batteries<br />
is lead. Billions of wet-cell lead-acid batteries are manufactured each year for<br />
use in automobiles, motorcycles and boats! That’s a lot of batteries, and a<br />
lot of pollutants! So until there is a better, environmentally friendly battery<br />
source, make sure you recycle and tell your friends to recycle too! Most city’s<br />
have drop-off centers for batteries. If you don’t know where to go, have a parent<br />
call the city offices to get the information you need. Remember to think<br />
GREEN SCIENCE!<br />
M. SETTING THE WATCH<br />
1. Setting the watch<br />
• Press A twice and the display will show the set month mode, then<br />
Press B to adjust to the right month.<br />
• After the month is set, Press A to confirm, and the set day mode<br />
will be displayed, Press B to adjust the to the right day.<br />
• After the day is set, Press A to confirm and the set hour<br />
mode will be displayed, Press B to adjust to the right<br />
hour.<br />
After the hour is set, Press A to confirm and the set<br />
• minute mode will be displayed, Press B to adjust<br />
to the right minute.<br />
After the minute is set, Press A to confirm and the<br />
• normal time will be displayed. You should see the two<br />
dots flashing between the hour and minute display.<br />
2. Viewing<br />
• By default, the clock display shows the current time.<br />
• To view the Date: Press B once. The clock display will resume showing the<br />
current time after 2 second.<br />
• To view the Seconds, Press B for twice. To resume to normal time, Press<br />
B again.<br />
• To view the Time and Date alternately, Press A once. To resume to normal<br />
time display, Press A 5 times to skip all set clock modes.<br />
N. TROUBLE SHOOTING<br />
If your experiment produces a weak sound or light signal, try one of<br />
the following:<br />
1. Give it time, the signal is sometimes weak at the beginning of the experiment,<br />
but gets stronger after a short while.<br />
2. You can try adding another connection to strengthen the current. For<br />
example in experiment 1, instead of using two potatoes, you could add<br />
another one. However, you will need to make another connection pair with<br />
an extra pair of zinc and copper plates. The whole circuit has to be connected<br />
in correct sequence. The display devices provided are of different<br />
voltage. The sound chip has the lowest voltage, whereas the clock is in<br />
the middle and the LED lamp is the highest. You will find the sound chip<br />
can easily be activated in most conditions. (You could even try using one<br />
connection for the sound chip by using a half lemon). However, the LED<br />
lamp, depending on the acidity of the solution and the metal used, may<br />
require as many as 3 or 4 connections to make it light up brightly.<br />
10 11
41-03261/1<br />
12<br />
N. TROUBLE SHOOTING (CONTINUED)<br />
3. Examine the metal plates for rust (oxidization). Use sand paper to remove<br />
any rust.<br />
4. Try putting the metal plates closer together (but not touching each other).<br />
Conduction will be better if the distance between the plates is shorter.<br />
5. If there is no reaction whatsoever, check all the connection points. Make<br />
sure that the connection points are correctly and firmly placed. Also,<br />
check if the polarities are correct - negative (black wires) and positive (red<br />
wires) terminals are connected properly.<br />
6. Check if the metal plates/wires are touching each other, this causes<br />
short circuits.<br />
QUESTIONS & COMMENTS<br />
We treasure you as a customer and your satisfaction with this product is important to us. In case<br />
you have any <strong>com</strong>ments or questions, or you find any parts of this kit missing or defective, please do<br />
not hesitate to contact our distributor in your country, whose address is printed on the package. You<br />
are also wel<strong>com</strong>e to contact our marketing support team at Email: infodesk@<strong>4M</strong>-<strong>IND</strong>.<strong>com</strong>, Fax (852)<br />
25911566, Tel (852) 28936241, Web site: WWW.<strong>4M</strong>-<strong>IND</strong>.COM<br />
Build a better tomorrow through education & awareness.<br />
You will like the other great Green Science kits:<br />
00-03263 Dynamo Torch<br />
Turn a simple toy motor into a generator that converts<br />
hand motion into electrical energy and powers a light<br />
bulb. No battery, no pollution, just amazement. The<br />
generator could be used as an awesome emergency<br />
torch. Caution: High Voltage Inspiration & Fun!<br />
00-03266 Robugs<br />
Millions of soda can are wasted everyday. Help save<br />
our environment. Recycle one of them and turn it into<br />
a cool robotic bug. Switch it on and watch it vibrate<br />
causing it to slide across the floor. It even emits a<br />
“buzz” as it moves along - just like a real bug. It’s an<br />
awesome robotic science kit.<br />
A. SAFETY MESSAGES<br />
1. Please read all instructions before you begin.<br />
2. Adult assistance and supervision is re<strong>com</strong>mended.<br />
3. The kit is intended for children age over 8.<br />
4. This kit and its finished products contain small parts which may cause<br />
choking hazard. Keep away from children under 3 years old.<br />
5. Please ask an adult for help when getting any material (i.e. potatoes,<br />
fruits, juice, etc) needed for the experiments.<br />
6. Food or beverages used in these experiments are not edible. Please<br />
dispose of them immediately after use.<br />
7. Do not connect any of the parts provided to any AC wall socket or any batteries.<br />
This may cause electric shock or a short circuit.<br />
B. CONTENTS<br />
4 zinc plates (silvery grey), 4 copper plates (brownish), 4 connection wires,<br />
2 plastic cups, 1 paper cup, 2 specially designed bottle screw caps, 1 light<br />
tower with LED lamp, 1 sound chip, 1 LCD watch moment with protective<br />
cover, 1 set transparent adhesive tapes, 1 set experiment instruction guide<br />
with experiment record sheet and fun facts.<br />
specially designed bottle screw caps<br />
Paper cup<br />
Plastic cups<br />
connection wires protective cover<br />
light tower with LED lamp<br />
LCD watch moment<br />
sound chip<br />
transparent adhesive tapes<br />
copper plates (brownish)<br />
zinc plates (silver grey)<br />
1