You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
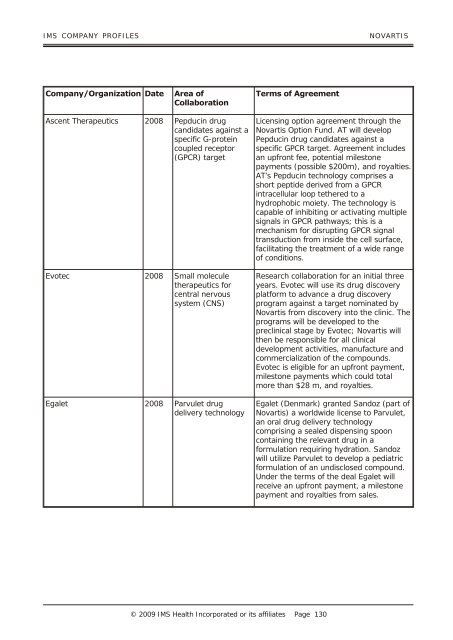
<strong>IMS</strong> COM PANY PRO FILES NOVARTIS<br />
<strong>Company</strong>/Organization Date Area of<br />
Collaboration<br />
Ascent Therapeutics 2008 Pepducin drug<br />
candidates against a<br />
specific G-protein<br />
coupled receptor<br />
(GPCR) target<br />
Evotec 2008 Small molecule<br />
therapeutics for<br />
central nervous<br />
system (CNS)<br />
Egalet 2008 Parvulet drug<br />
delivery technology<br />
Terms of Agreement<br />
© 2009 <strong>IMS</strong> Health In cor po rated or its af fil i ates Page 130<br />
Licensing option agreement through the<br />
Novartis Option Fund. AT will develop<br />
Pepducin drug candidates against a<br />
specific GPCR target. Agreement includes<br />
an upfront fee, potential milestone<br />
payments (possible $200m), and royalties.<br />
AT’s Pepducin technology comprises a<br />
short peptide derived from a GPCR<br />
intracellular loop tethered to a<br />
hydrophobic moiety. The technology is<br />
capable of inhibiting or activating multiple<br />
signals in GPCR pathways; this is a<br />
mechanism for disrupting GPCR signal<br />
transduction from inside the cell surface,<br />
facilitating the treatment of a wide range<br />
of conditions.<br />
Research collaboration for an initial three<br />
years. Evotec will use its drug discovery<br />
platform to advance a drug discovery<br />
program against a target nominated by<br />
Novartis from discovery into the clinic. The<br />
programs will be developed to the<br />
preclinical stage by Evotec; Novartis will<br />
then be responsible for all clinical<br />
development activities, manufacture and<br />
commercialization of the compounds.<br />
Evotec is eligible for an upfront payment,<br />
milestone payments which could total<br />
more than $28 m, and royalties.<br />
Egalet (Denmark) granted Sandoz (part of<br />
Novartis) a worldwide license to Parvulet,<br />
an oral drug delivery technology<br />
comprising a sealed dispensing spoon<br />
containing the relevant drug in a<br />
formulation requiring hydration. Sandoz<br />
will utilize Parvulet to develop a pediatric<br />
formulation of an undisclosed compound.<br />
Under the terms of the deal Egalet will<br />
receive an upfront payment, a milestone<br />
payment and royalties from sales.