BULETINUL INSTITUTULUI POLITEHNIC DIN IAŞI - Universitatea ...
BULETINUL INSTITUTULUI POLITEHNIC DIN IAŞI - Universitatea ...
BULETINUL INSTITUTULUI POLITEHNIC DIN IAŞI - Universitatea ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
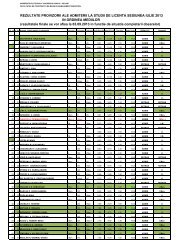
Bul. Inst. Polit. Iaşi, t. LVI (LX), f. 2, 2010 109<br />
3. The surface of Anodized Aluminium<br />
Aluminium alloys and anodized aluminium will theoretically have a high<br />
surface tension, so wetting of the surface with an adhesive would not be a<br />
problem, because the adhesives will have a surface tension much lower.<br />
Aluminium and anodized aluminium will react with CO2 and possible<br />
contaminations in the air and will there fore in practical have a surface tension,<br />
which is so low that wetting with the adhesives is not possible, because the<br />
actually surface tension is lower for aluminium surface than for the adhesive.<br />
If the surface is new created the surface tension will still be high and wetting<br />
will not be a problem. For solving the problem with not anodized aluminium the<br />
aluminium surface is often chromatized or phosphatized to obtain a good wetting.<br />
The surface of anodized aluminium itself is possible to wet with an adhesive<br />
especially if it is new treated and that will say that as soon after the anodizing the<br />
wetting will take place the better adhesion will be obtained. The bigger surface<br />
the better wetting is possible and that means also that if the anodized surface is<br />
used for bonding before sealing. The fresh anodized aluminium surface is the best<br />
surface for adhesive bonding, because the surface there has reactive groups,<br />
which will give possibility for chemical bonding in the interphase. The reactive<br />
groups give a bigger contribution to the surface tension because of more hydrogen<br />
bonds and the physical adsorption in the interphase will increase. The mechanical<br />
interlocking will also increase because of the porousity in the anodized surface.<br />
After sealing the surface is like un-anodized aluminium and the advantages<br />
obtained by the anodizing will disappear for the bonding.<br />
4. Surface Treatments<br />
Before bonding the surface need to be clean. The surface shall as mentioned<br />
above be with so many reactive groups as possible and the surface tension shall<br />
be as big as possible. The possibility for mechanical interlocking shall be<br />
present if possible.<br />
If the surface is contaminated with oil or grease from production or cutting<br />
process the surface tension will be the same as the surface tension of oil and<br />
grease, which will say very low, because those materials will almost have a<br />
contribution form the dispersion forces, very few if any from the polar forces<br />
and no from the hydrogen forces. Such a surface is impossible to bond on. The<br />
grease and oil shall be removed with an organic solvent as f.ex. isopropyl<br />
alcohol or CO2. The surface can if it is non anodized aluminium or is a sealed<br />
anodized aluminium be treated with a Scotch Brite for obtaining a bigger<br />
surface for mechanical interlocking. The treatment with Scotch Brite shall take<br />
place before the cleaning for removing dust from the surface.