You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2.6.1 Metal–Carbene Complexes 97<br />
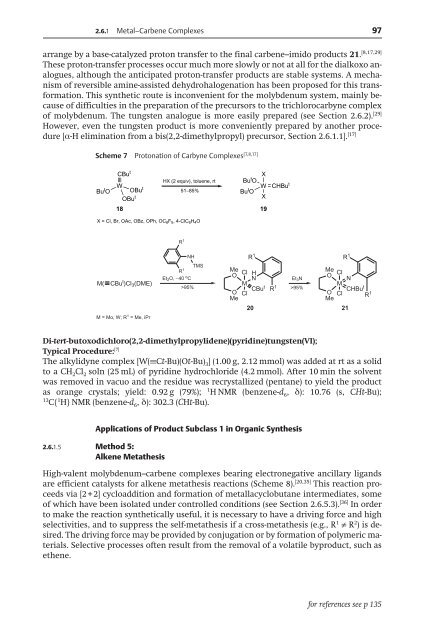
arrange by a base-catalyzed proton transfer to the final carbene–imido products 21. [8,17,29]<br />
These proton-transfer processes occur much more slowly or not at all for the dialkoxo analogues,<br />
although the anticipated proton-transfer products are stable systems. A mechanism<br />
of reversible amine-assisted dehydrohalogenation has been proposed for this transformation.<br />
This synthetic route is inconvenient for the molybdenum system, mainly because<br />
of difficulties in the preparation of the precursors to the trichlorocarbyne complex<br />
of molybdenum. The tungsten analogue is more easily prepared (see Section 2.6.2). [29]<br />
However, even the tungsten product is more conveniently prepared by another procedure<br />
[Æ-H elimination from a bis(2,2-dimethylpropyl) precursor, Section 2.6.1.1]. [17]<br />
Scheme 7 Protonation of Carbyne Complexes [7,8,17]<br />
Bu t O<br />
CBu t<br />
W<br />
OBut OBut 18<br />
HX (2 equiv), toluene, rt<br />
51−85%<br />
X = Cl, Br, OAc, OBz, OPh, OC 6F 5, 4-ClC 6H 4O<br />
M( CBu t )Cl 3(DME)<br />
M = Mo, W; R 1 = Me, iPr<br />
R 1<br />
NH<br />
R1 Et2O, −40 o TMS<br />
C<br />
>95%<br />
X<br />
Bu<br />
W<br />
t Bu<br />
O<br />
tO X<br />
CHBut R 1<br />
19<br />
Cl<br />
N<br />
M<br />
CBu<br />
Cl<br />
t R1 Me<br />
O H<br />
O<br />
Me<br />
20<br />
Et3N<br />
>95%<br />
R 1<br />
Cl<br />
N<br />
M<br />
CHBu<br />
Cl<br />
t<br />
R1 Me<br />
O<br />
O<br />
Me<br />
Di-tert-butoxodichloro(2,2-dimethylpropylidene)(pyridine)tungsten(VI);<br />
Typical Procedure: [7]<br />
The alkylidyne complex [W(”Ct-Bu)(Ot-Bu) 3] (1.00 g, 2.12 mmol) was added at rt as a solid<br />
to a CH 2Cl 2 soln (25 mL) of pyridine hydrochloride (4.2 mmol). After 10 min the solvent<br />
was removed in vacuo and the residue was recrystallized (pentane) to yield the product<br />
as orange crystals; yield: 0.92 g (79%); 1 H NMR (benzene-d 6, ä): 10.76 (s, CHt-Bu);<br />
13 C{ 1 H} NMR (benzene-d6, ä): 302.3 (CHt-Bu).<br />
Applications of Product Subclass 1 in Organic Synthesis<br />
2.6.1.5 Method 5:<br />
Alkene Metathesis<br />
High-valent molybdenum–carbene complexes bearing electronegative ancillary ligands<br />
are efficient catalysts for alkene metathesis reactions (Scheme 8). [20,35] This reaction proceeds<br />
via [2 +2] cycloaddition and formation of metallacyclobutane intermediates, some<br />
of which have been isolated under controlled conditions (see Section 2.6.5.3). [36] In order<br />
to make the reaction synthetically useful, it is necessary to have a driving force and high<br />
selectivities, and to suppress the self-metathesis if a cross-metathesis (e.g., R 1 „ R 2 ) is desired.<br />
The driving force may be provided by conjugation or by formation of polymeric materials.<br />
Selective processes often result from the removal of a volatile byproduct, such as<br />
ethene.<br />
21<br />
for references see p 135