Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1.1.4 Nickel–Alkene Complexes 65<br />
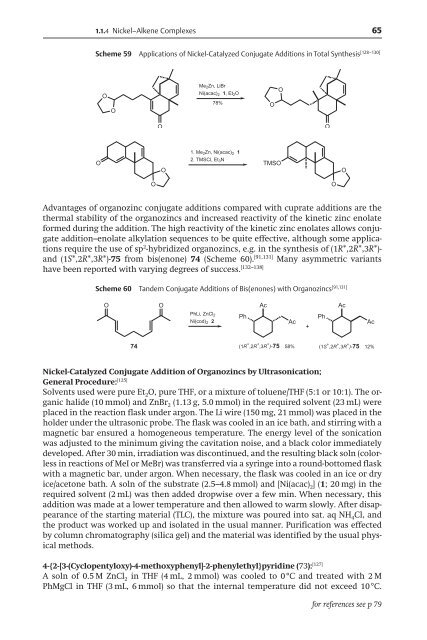
Scheme 59 Applications of Nickel-Catalyzed Conjugate Additions in Total Synthesis [128–130]<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
Me 2Zn, LiBr<br />
Ni(acac) 2 1, Et2O<br />
78%<br />
1. Me2Zn, Ni(acac)2 1<br />
2. TMSCl, Et 3N<br />
O<br />
O<br />
TMSO<br />
Advantages of organozinc conjugate additions compared with cuprate additions are the<br />
thermal stability of the organozincs and increased reactivity of the kinetic zinc enolate<br />
formed during the addition. The high reactivity of the kinetic zinc enolates allows conjugate<br />
addition–enolate alkylation sequences to be quite effective, although some applications<br />
require the use of sp 2 -hybridized organozincs, e.g. in the synthesis of (1R*,2R*,3R*)and<br />
(1S*,2R*,3R*)-75 from bis(enone) 74 (Scheme 60). [91,131] Many asymmetric variants<br />
have been reported with varying degrees of success. [132–138]<br />
Scheme 60 Tandem Conjugate Additions of Bis(enones) with Organozincs [91,131]<br />
O O<br />
74<br />
PhLi, ZnCl2<br />
Ni(cod)2 2<br />
Ph<br />
Ac<br />
Ac<br />
(1R ∗ ,2R ∗ ,3R ∗ )-75 58%<br />
+<br />
Ph<br />
O<br />
O<br />
O<br />
Ac<br />
Ac<br />
(1S ∗ ,2R ∗ ,3R ∗ )-75 12%<br />
Nickel-Catalyzed Conjugate Addition of Organozincs by Ultrasonication;<br />
General Procedure: [125]<br />
Solvents used were pure Et 2O, pure THF, or a mixture of toluene/THF (5:1 or 10:1). The organic<br />
halide (10 mmol) and ZnBr 2 (1.13 g, 5.0 mmol) in the required solvent (23 mL) were<br />
placed in the reaction flask under argon. The Li wire (150 mg, 21 mmol) was placed in the<br />
holder under the ultrasonic probe. The flask was cooled in an ice bath, and stirring with a<br />
magnetic bar ensured a homogeneous temperature. The energy level of the sonication<br />
was adjusted to the minimum giving the cavitation noise, and a black color immediately<br />
developed. After 30 min, irradiation was discontinued, and the resulting black soln (colorless<br />
in reactions of MeI or MeBr) was transferred via a syringe into a round-bottomed flask<br />
with a magnetic bar, under argon. When necessary, the flask was cooled in an ice or dry<br />
ice/acetone bath. A soln of the substrate (2.5–4.8mmol) and [Ni(acac) 2](1; 20 mg) in the<br />
required solvent (2 mL) was then added dropwise over a few min. When necessary, this<br />
addition was made at a lower temperature and then allowed to warm slowly. After disappearance<br />
of the starting material (TLC), the mixture was poured into sat. aq NH 4Cl, and<br />
the product was worked up and isolated in the usual manner. Purification was effected<br />
by column chromatography (silica gel) and the material was identified by the usual physical<br />
methods.<br />
4-{2-[3-(Cyclopentyloxy)-4-methoxyphenyl]-2-phenylethyl}pyridine (73): [127]<br />
A soln of 0.5 M ZnCl 2 in THF (4 mL, 2 mmol) was cooled to 08C and treated with 2 M<br />
PhMgCl in THF (3 mL, 6 mmol) so that the internal temperature did not exceed 108C.<br />
for references see p 79