Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
46 Science of Synthesis 1.1 Organometallic Complexes of Nickel<br />
with CH 2Cl 2 (3 ” 35 mL). The combined organic layers were dried (MgSO 4). Removal of the<br />
drying agent through filtration, followed by solvent evaporation in vacuo, afforded a yellow<br />
oil. Chromatography (silica gel, hexanes/EtOAc 15:1) afforded 38 as a colorless oil;<br />
yield: 69 mg (73%).<br />
1.1.2.7.2 Variation2:<br />
Enal-Derived ð-Allyl Complexes<br />
Enal-derived nickel–ð-allyl complexes are efficient partners in cross-coupling reactions<br />
with alkenyl- and arylstannanes leading to products such as 39 (Scheme 23). [39] As described<br />
for the allylic ether derived complexes, the mechanism of the coupling process<br />
involves transmetalation followed by reductive elimination. The reaction is catalytic in<br />
nickel, unlike couplings of enals with alkyl halides which require stoichiometric quantities<br />
of nickel. Many other variants of nickel-catalyzed conjugate additions may involve ðallyl<br />
complexes, but these are treated separately in a section on alkenes (Section 1.1.4.2)<br />
because the mechanism is poorly defined in most nickel-catalyzed conjugate additions.<br />
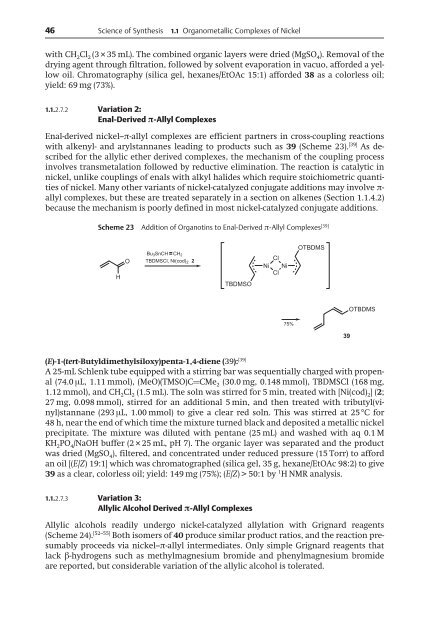
Scheme 23 Addition of Organotins to Enal-Derived ð-Allyl Complexes [39]<br />
H<br />
O<br />
Bu3SnCH CH2 TBDMSCl, Ni(cod)2 2<br />
TBDMSO<br />
Cl<br />
Ni Ni<br />
Cl<br />
75%<br />
OTBDMS<br />
39<br />
OTBDMS<br />
(E)-1-(tert-Butyldimethylsiloxy)penta-1,4-diene (39): [39]<br />
A 25-mL Schlenk tube equipped with a stirring bar was sequentially charged with propenal<br />
(74.0 ìL, 1.11 mmol), (MeO)(TMSO)C=CMe 2 (30.0 mg, 0.148mmol), TBDMSCl (168mg,<br />
1.12 mmol), and CH 2Cl 2 (1.5 mL). The soln was stirred for 5 min, treated with [Ni(cod) 2](2;<br />
27 mg, 0.098mmol), stirred for an additional 5 min, and then treated with tributyl(vinyl)stannane<br />
(293 ìL, 1.00 mmol) to give a clear red soln. This was stirred at 258C for<br />
48h, near the end of which time the mixture turned black and deposited a metallic nickel<br />
precipitate. The mixture was diluted with pentane (25 mL) and washed with aq 0.1 M<br />
KH 2PO 4/NaOH buffer (2 ” 25 mL, pH 7). The organic layer was separated and the product<br />
was dried (MgSO 4), filtered, and concentrated under reduced pressure (15 Torr) to afford<br />
an oil [(E/Z) 19:1] which was chromatographed (silica gel, 35 g, hexane/EtOAc 98:2) to give<br />
39 as a clear, colorless oil; yield: 149 mg (75%); (E/Z) > 50:1 by 1 H NMR analysis.<br />
1.1.2.7.3 Variation3:<br />
Allylic Alcohol Derived ð-Allyl Complexes<br />
Allylic alcohols readily undergo nickel-catalyzed allylation with Grignard reagents<br />
(Scheme 24). [52–55] Both isomers of 40 produce similar product ratios, and the reaction presumably<br />
proceeds via nickel–ð-allyl intermediates. Only simple Grignard reagents that<br />
lack â-hydrogens such as methylmagnesium bromide and phenylmagnesium bromide<br />
are reported, but considerable variation of the allylic alcohol is tolerated.