Detailed table of contents (pdf)
Detailed table of contents (pdf)
Detailed table of contents (pdf)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
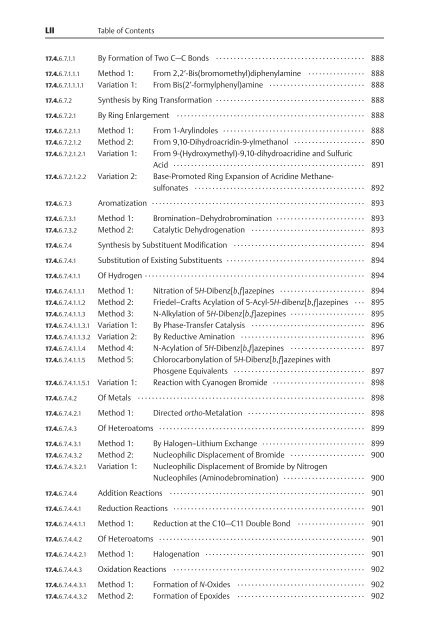
LII Table <strong>of</strong> Contents<br />
17.4.6.7.1.1 By Formation <strong>of</strong> Two C—C Bonds .......................................... 888<br />
17.4.6.7.1.1.1 Method 1: From 2,2¢-Bis(bromomethyl)diphenylamine ................ 888<br />
17.4.6.7.1.1.1.1 Variation 1: From Bis(2¢-formylphenyl)amine ........................... 888<br />
17.4.6.7.2 Synthesis by Ring Transformation .......................................... 888<br />
17.4.6.7.2.1 By Ring Enlargement ..................................................... 888<br />
17.4.6.7.2.1.1 Method 1: From 1-Arylindoles ........................................ 888<br />
17.4.6.7.2.1.2 Method 2: From 9,10-Dihydroacridin-9-ylmethanol .................... 890<br />
17.4.6.7.2.1.2.1 Variation 1: From 9-(Hydroxymethyl)-9,10-dihydroacridine and Sulfuric<br />
Acid ...................................................... 891<br />
17.4.6.7.2.1.2.2 Variation 2: Base-Promoted Ring Expansion <strong>of</strong> Acridine Methanesulfonates<br />
................................................ 892<br />
17.4.6.7.3 Aromatization ............................................................ 893<br />
17.4.6.7.3.1 Method 1: Bromination–Dehydrobromination ......................... 893<br />
17.4.6.7.3.2 Method 2: Catalytic Dehydrogenation ................................ 893<br />
17.4.6.7.4 Synthesis by Substituent Modification ..................................... 894<br />
17.4.6.7.4.1 Substitution <strong>of</strong> Existing Substituents ....................................... 894<br />
17.4.6.7.4.1.1 Of Hydrogen .............................................................. 894<br />
17.4.6.7.4.1.1.1 Method 1: Nitration <strong>of</strong> 5H-Dibenz[b,f]azepines ........................ 894<br />
17.4.6.7.4.1.1.2 Method 2: Friedel–Crafts Acylation <strong>of</strong> 5-Acyl-5H-dibenz[b,f]azepines ... 895<br />
17.4.6.7.4.1.1.3 Method 3: N-Alkylation <strong>of</strong> 5H-Dibenz[b,f]azepines ..................... 895<br />
17.4.6.7.4.1.1.3.1 Variation 1: By Phase-Transfer Catalysis ................................ 896<br />
17.4.6.7.4.1.1.3.2 Variation 2: By Reductive Amination ................................... 896<br />
17.4.6.7.4.1.1.4 Method 4: N-Acylation <strong>of</strong> 5H-Dibenz[b,f]azepines ..................... 897<br />
17.4.6.7.4.1.1.5 Method 5: Chlorocarbonylation <strong>of</strong> 5H-Dibenz[b,f]azepines with<br />
Phosgene Equivalents ..................................... 897<br />
17.4.6.7.4.1.1.5.1 Variation 1: Reaction with Cyanogen Bromide .......................... 898<br />
17.4.6.7.4.2 Of Metals ................................................................ 898<br />
17.4.6.7.4.2.1 Method 1: Directed ortho-Metalation ................................. 898<br />
17.4.6.7.4.3 Of Heteroatoms .......................................................... 899<br />
17.4.6.7.4.3.1 Method 1: By Halogen–Lithium Exchange ............................. 899<br />
17.4.6.7.4.3.2 Method 2: Nucleophilic Displacement <strong>of</strong> Bromide ..................... 900<br />
17.4.6.7.4.3.2.1 Variation 1: Nucleophilic Displacement <strong>of</strong> Bromide by Nitrogen<br />
Nucleophiles (Aminodebromination) ....................... 900<br />
17.4.6.7.4.4 Addition Reactions ....................................................... 901<br />
17.4.6.7.4.4.1 Reduction Reactions ...................................................... 901<br />
17.4.6.7.4.4.1.1 Method 1: Reduction at the C10—C11 Double Bond ................... 901<br />
17.4.6.7.4.4.2 Of Heteroatoms .......................................................... 901<br />
17.4.6.7.4.4.2.1 Method 1: Halogenation ............................................. 901<br />
17.4.6.7.4.4.3 Oxidation Reactions ...................................................... 902<br />
17.4.6.7.4.4.3.1 Method 1: Formation <strong>of</strong> N-Oxides .................................... 902<br />
17.4.6.7.4.4.3.2 Method 2: Formation <strong>of</strong> Epoxides .................................... 902