d(GC) - Association of Biotechnology and Pharmacy
d(GC) - Association of Biotechnology and Pharmacy
d(GC) - Association of Biotechnology and Pharmacy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Current Trends in <strong>Biotechnology</strong> <strong>and</strong> <strong>Pharmacy</strong><br />
Vol. 6 (2) 229-240 April 2012, ISSN 0973-8916 (Print), 2230-7303 (Online)<br />
30, 33). According to our experiments, an<br />
appropriate time for IFN PEGylation was 2 h (data<br />
not shown). The evaluated variables <strong>and</strong> their<br />
levels are showed in Table 1. Yates table design<br />
is presented in Table 2. All <strong>of</strong> the experiments<br />
were performed in duplicate, unless stated<br />
otherwise.<br />
Design <strong>of</strong> experiments, Taguchi statistical<br />
design: Taguchi statistical design was selected<br />
to determine the optimum conditions for IFN<br />
PEGylation, using 5 <strong>and</strong> 20 kDa linear mPEG-<br />
SPAs (34). An L 9 Taguchi array (34) was designed<br />
based on three variables (determined from the<br />
previous design) at three levels (Table 3) (30).<br />
The design (L 9 array) is shown in Table 4. All <strong>of</strong><br />
the experiments were performed in duplicate<br />
unless stated otherwise.<br />
Design <strong>of</strong> experiments, one factor at a time<br />
design: One factor at a time design was selected<br />
to study the impact <strong>of</strong> one variable (the ratio <strong>of</strong><br />
protein to mPEG fractions) on single response<br />
(HPLC or Ninhydrin test) for PEGylated IFN using<br />
40 kDa branched mPEG-SPA (32). To obtain the<br />
optimum conditions for PEGylation <strong>of</strong> IFN with<br />
40 kDa branched mPEG-SPA, the effect <strong>of</strong> the<br />
ratio <strong>of</strong> protein to mPEG fractions (at values <strong>of</strong><br />
1:10, 1:20, 1:30 <strong>and</strong> 1:40) at two different pHs <strong>of</strong><br />
7.4 <strong>and</strong> 8.0 was investigated.<br />
Determination <strong>of</strong> antiviral activity: The specific<br />
antiviral activity <strong>of</strong> IFN-ß- 1a was determined<br />
using a cytopathic effect (CPE) bioassay that<br />
232<br />
measures the ability <strong>of</strong> proteins to protect HeLa<br />
cells (grown at 37 °C/5% CO 2 ) challenged with<br />
the Vesicular Stomatitis Virus (VSV). HeLa cells<br />
suspended in 10 mL <strong>of</strong> Dulbecco´s modified<br />
eagles medium (DMEM) containing 5% fetal<br />
bovine serum, 9.53 g/L DMEM medium, 0.1 g/L<br />
<strong>of</strong> sodium pyruvate, 0.3 g/L <strong>of</strong> L-glutamine, 2.5-<br />
3.5 g/L <strong>of</strong> sodium bicarbonate <strong>and</strong> 50 µg/mL <strong>of</strong><br />
kanamycin-neomycin, were added to a 96-well<br />
microtiter plate <strong>and</strong> incubated for 24 h at 37° C.<br />
Serial dilutions <strong>of</strong> duplicate IFN-β-1a st<strong>and</strong>ards<br />
<strong>and</strong> test samples were then added, <strong>and</strong> the cells<br />
were incubated for further 24 h. The medium was<br />
removed, <strong>and</strong> VSV virus was added. The cells<br />
were incubated for 48 h, then 50 µL <strong>of</strong> 5 mg/mL<br />
<strong>of</strong> 3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl-2Htetrazolium<br />
bromide (MTT) in PBS was added.<br />
After 1 h <strong>of</strong> incubation, the medium was removed<br />
<strong>and</strong> the wells were washed with 100 µL <strong>of</strong> PBS.<br />
The cells <strong>and</strong> dye were then solubilized with 100<br />
µL <strong>of</strong> 1.2 N HCl in 2-propanol, <strong>and</strong> the optical<br />
absorbance <strong>of</strong> medium was then measured at<br />
570 nm (1).<br />
Results <strong>and</strong> discussion<br />
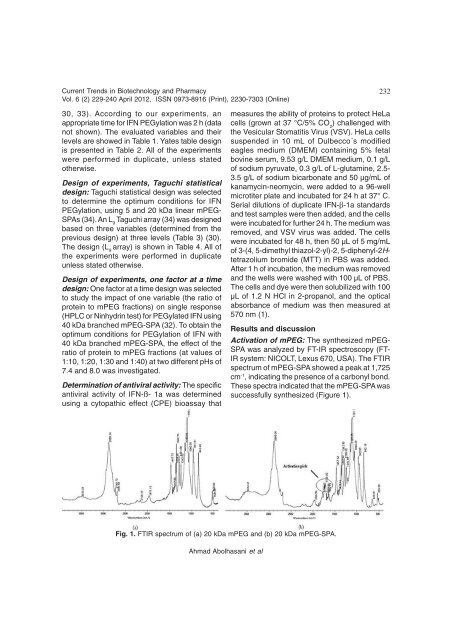
Activation <strong>of</strong> mPEG: The synthesized mPEG-<br />
SPA was analyzed by FT-IR spectroscopy (FT-<br />
IR system: NICOLT, Lexus 670, USA). The FTIR<br />
spectrum <strong>of</strong> mPEG-SPA showed a peak at 1,725<br />
cm-1 , indicating the presence <strong>of</strong> a carbonyl bond.<br />
These spectra indicated that the mPEG-SPA was<br />
successfully synthesized (Figure 1).<br />
Fig. 1. FTIR spectrum <strong>of</strong> (a) 20 kDa mPEG <strong>and</strong> (b) 20 kDa mPEG-SPA.<br />
Ahmad Abolhasani et al