2010 American Heart Association
2010 American Heart Association
2010 American Heart Association
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
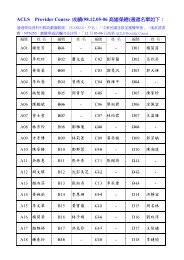
S896 Circulation November 2, <strong>2010</strong><br />
Guidelines Part 14: PALS Writing Group Disclosures, Continued<br />
Writing<br />
Group<br />
Member Employment Research Grant<br />
Ericka L.<br />
Fink<br />
Eugene B.<br />
Freid<br />
Robert W.<br />
Hickey<br />
Bradley S.<br />
Marino<br />
Vinay M.<br />
Nadkarni<br />
Lester T.<br />
Proctor<br />
Faiqa A.<br />
Qureshi<br />
Kennith<br />
Sartorelli<br />
Alexis<br />
Topjian<br />
Elise W. van<br />
der Jagt<br />
Arno L.<br />
Zaritsky<br />
Children’s Hospital of Pittsburgh<br />
of UPMC–Assistant Professor<br />
Nemours Childrens<br />
Clinics–Anesthesiologist and<br />
Intensivist<br />
University of Pittsburgh–Pediatric<br />
Emergency Medicine Physician<br />
Cincinnati Children’s Hospital<br />
Medical Center–Associate<br />
Professor of Pediatrics<br />
University of Pennsylvania,<br />
Children’s Hospital of<br />
Philadelphia–Attending Physician,<br />
Pediatric Critical Care<br />
University of Wisconsin-Madison<br />
College of Medicine and Public<br />
Health–Professor<br />
Children’s Specialty<br />
Group—Partner<br />
University of Vermont–Associate<br />
Professor of Surgery<br />
University of<br />
Pennsylvania–Assistant Professor<br />
University of Rochester–Professor<br />
of Pediatrics and Critical Care<br />
Childen’s Hospital of The King’s<br />
Daughters-Sr. VP for Clinical<br />
Services<br />
Other<br />
Research<br />
Support Speakers’ Bureau/ Honoraria<br />
Ownership<br />
Interest<br />
Consultant/ Advisory<br />
Board Other<br />
†National Institutes of<br />
Health, NINDS K23, Laerdal<br />
Foundation, and Children’s<br />
Hospital of Pittsburgh<br />
Clinical and Translational<br />
Science Institute grants to<br />
study duration of<br />
hypothermia after pediatric<br />
cardiac arrest.<br />
None None None None None<br />
None None *$1500.00 from University of North<br />
Carolina to Nemours Childrens<br />
Clinics for 3 lectures at annual<br />
anesthesiology conferencelectures<br />
related to anesthesia<br />
management of patients with<br />
cancer, operating room ventilators<br />
& postoperative nausea/vomiting.<br />
No direct conflicts with Pediatric<br />
Life support topics<br />
None None None<br />
†NIH sponsored research on<br />
the effect of cyclopentenone<br />
prostaglandins upon<br />
post-ischemic brain.<br />
None None None None *Occasional expert<br />
witness (1–2 times<br />
per year)<br />
None None None None None None<br />
†NIH RO1: Coinvestigator,<br />
Therapeutic Hypothermia<br />
After Pediatric Cardiac<br />
Arrest Center of Excellence<br />
Grant, PI, Laerdal<br />
Foundation for Acute Care<br />
Medicine AHRQ: Agency for<br />
Healthcare Research and<br />
Quality: PI, Tracheal<br />
Intubation Safety in<br />
Pediatric ICUs<br />
*NHTSA: Coinvestigator,<br />
Chest compression<br />
characteristics in children<br />
None None None None *Volunteer (no<br />
salary or<br />
remuneration),<br />
World Federation<br />
of Pediatric<br />
Intensive and<br />
Critical Care<br />
Societies<br />
Volunteer (no<br />
salary), Data Safety<br />
and Monitoring<br />
Board, CIRC study<br />
None None None None None None<br />
None None None None None None<br />
None None None None None None<br />
*Site principal investigator<br />
at the Children’s hospital of<br />
Philadelphia for the<br />
“Therpaeutic Hypothermia<br />
after Pediatric Cardiac<br />
Arrest� funded via an NIH<br />
U01<br />
None None None None None<br />
None None None None None None<br />
None None None None *Data Safety Monitoring<br />
Board for NIH-funded<br />
pediatric hypothermia<br />
after cardiac arrest<br />
research project<br />
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure<br />
Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000<br />
or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns<br />
$10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.<br />
*Modest.<br />
†Significant.<br />
Downloaded from<br />
circ.ahajournals.org at NATIONAL TAIWAN UNIV on October 18, <strong>2010</strong><br />
None