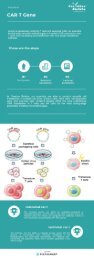
Application of CD19-CART cells in B-cell lymphocytic leukemia
CD19 CART cells can recognize specific CD19 targets of B-cell lymphocytic leukemia. By releasing cytokines such as perforin and granzymes, CD19-CART cells attack the B lymphocytes expressing CD19 antigen, thereby prompting the body to eliminate malignant lymphocytes. The US Sloan-Kettering Cancer Center applied autologous 19-28z CART in the treatment of refractory and relapsed B-ALL. Fourteen of the 16 patients had CR and were even effective in relapsed Ph+ ALL after transplantation. Treatment with CART also creates conditions for allogeneic hematopoietic stem cell transplantation. The University of Pennsylvania also reported the use of 19-CD137zCART for the treatment of B-cell tumors. Thirty patients with refractory and relapsed B-ALL and 27 patients achieved CR. The 6-month disease-free survival rate was 67%, and the overall survival rate was 78%.
CD19 CART cells can recognize specific CD19 targets of B-cell lymphocytic leukemia. By releasing cytokines such as perforin and granzymes, CD19-CART cells attack the B lymphocytes expressing CD19 antigen, thereby prompting the body to eliminate malignant lymphocytes. The US Sloan-Kettering Cancer Center applied autologous 19-28z CART in the treatment of refractory and relapsed B-ALL. Fourteen of the 16 patients had CR and were even effective in relapsed Ph+ ALL after transplantation. Treatment with CART also creates conditions for allogeneic hematopoietic stem cell transplantation. The University of Pennsylvania also reported the use of 19-CD137zCART for the treatment of B-cell tumors. Thirty patients with refractory and relapsed B-ALL and 27 patients achieved CR. The 6-month disease-free survival rate was 67%, and the overall survival rate was 78%.
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
f<strong>in</strong>d out the optimal number <strong>of</strong> <strong>CART</strong> <strong><strong>cell</strong>s</strong> for anti-tumor effects. In this study, 10 patients with<br />
relapsed and refractory CLL were enrolled. All patients were treated with <strong>CART</strong> <strong><strong>cell</strong>s</strong>, <strong>of</strong> whom 6<br />
were treated with low amounts (5×107) and 4 patients were treated with high amounts (5×108).<br />
The median follow-up was 3 months. There was no significant difference <strong>in</strong> the efficacy and<br />
adverse reactions between the two groups.<br />
The use <strong>of</strong> <strong>CART</strong> <strong><strong>cell</strong>s</strong> to specifically target <strong>CD19</strong>-positive B-<strong>cell</strong> lymphoma and <strong>leukemia</strong><br />
patients has achieved significant cl<strong>in</strong>ical efficacy and is expected to be widely used. Although the<br />
current <strong>CART</strong> treatment is carried out for a short period <strong>of</strong> time, there is still a lack <strong>of</strong> large-scale<br />
samples, and many adverse reactions and complications need to be gradually discovered and<br />
resolved <strong>in</strong> cl<strong>in</strong>ical trials. However, <strong>in</strong> the cl<strong>in</strong>ical trials reported for the treatment <strong>of</strong> B-<strong>cell</strong><br />
lymphoma and <strong>leukemia</strong>, <strong>CART</strong> showed a very significant effect, especially for relapsed and<br />
refractory patients, thus provid<strong>in</strong>g a basis for a larger-scale, multi-center cl<strong>in</strong>ical study.<br />
Author Bio<br />
As a global company, Creative Biolabs has more than 200 talented and well-tra<strong>in</strong>ed scientists<br />
located <strong>in</strong> different cont<strong>in</strong>ents work<strong>in</strong>g closely with partners from the entire world to develop and<br />
produce medic<strong>in</strong>es <strong>of</strong> tomorrow. Specifically, we are the established lead<strong>in</strong>g expert <strong>in</strong> TCR and<br />
CAR T&NK <strong>cell</strong> immune therapy development, as we <strong>of</strong>fer the one-stop custom services that<br />
cover the entire new drug development pipel<strong>in</strong>e. Additionally, we also <strong>of</strong>fer an exclusive l<strong>in</strong>e <strong>of</strong><br />
ready-to-use TCR T and CAR T&NK <strong>cell</strong> construction products, such as virus packag<strong>in</strong>g,<br />
purification, expansion and titer determ<strong>in</strong>ation kits. Furthermore, we have built up a unique<br />
unparalleled CAR construction and production platform for all four CAR generations.