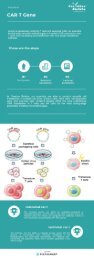
Application of CD19-CART cells in B-cell lymphocytic leukemia
CD19 CART cells can recognize specific CD19 targets of B-cell lymphocytic leukemia. By releasing cytokines such as perforin and granzymes, CD19-CART cells attack the B lymphocytes expressing CD19 antigen, thereby prompting the body to eliminate malignant lymphocytes. The US Sloan-Kettering Cancer Center applied autologous 19-28z CART in the treatment of refractory and relapsed B-ALL. Fourteen of the 16 patients had CR and were even effective in relapsed Ph+ ALL after transplantation. Treatment with CART also creates conditions for allogeneic hematopoietic stem cell transplantation. The University of Pennsylvania also reported the use of 19-CD137zCART for the treatment of B-cell tumors. Thirty patients with refractory and relapsed B-ALL and 27 patients achieved CR. The 6-month disease-free survival rate was 67%, and the overall survival rate was 78%.
CD19 CART cells can recognize specific CD19 targets of B-cell lymphocytic leukemia. By releasing cytokines such as perforin and granzymes, CD19-CART cells attack the B lymphocytes expressing CD19 antigen, thereby prompting the body to eliminate malignant lymphocytes. The US Sloan-Kettering Cancer Center applied autologous 19-28z CART in the treatment of refractory and relapsed B-ALL. Fourteen of the 16 patients had CR and were even effective in relapsed Ph+ ALL after transplantation. Treatment with CART also creates conditions for allogeneic hematopoietic stem cell transplantation. The University of Pennsylvania also reported the use of 19-CD137zCART for the treatment of B-cell tumors. Thirty patients with refractory and relapsed B-ALL and 27 patients achieved CR. The 6-month disease-free survival rate was 67%, and the overall survival rate was 78%.
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Application</strong> <strong>of</strong> <strong>CD19</strong>-<strong>CART</strong> <strong><strong>cell</strong>s</strong> <strong>in</strong> B-<strong>cell</strong> <strong>lymphocytic</strong> <strong>leukemia</strong><br />
<strong>CD19</strong> <strong>CART</strong> <strong><strong>cell</strong>s</strong> can recognize specific <strong>CD19</strong> targets <strong>of</strong> B-<strong>cell</strong> <strong>lymphocytic</strong> <strong>leukemia</strong>. By<br />
releas<strong>in</strong>g cytok<strong>in</strong>es such as perfor<strong>in</strong> and granzymes, <strong>CD19</strong>-<strong>CART</strong> <strong><strong>cell</strong>s</strong> attack the B lymphocytes<br />
express<strong>in</strong>g <strong>CD19</strong> antigen, thereby prompt<strong>in</strong>g the body to elim<strong>in</strong>ate malignant lymphocytes. The<br />
US Sloan-Ketter<strong>in</strong>g Cancer Center applied autologous 19-28z <strong>CART</strong> <strong>in</strong> the treatment <strong>of</strong> refractory<br />
and relapsed B-ALL. Fourteen <strong>of</strong> the 16 patients had CR and were even effective <strong>in</strong> relapsed Ph+<br />
ALL after transplantation. Treatment with <strong>CART</strong> also creates conditions for allogeneic<br />
hematopoietic stem <strong>cell</strong> transplantation. The University <strong>of</strong> Pennsylvania also reported the use <strong>of</strong><br />
19-CD137z<strong>CART</strong> for the treatment <strong>of</strong> B-<strong>cell</strong> tumors. Thirty patients with refractory and relapsed<br />
B-ALL and 27 patients achieved CR. The 6-month disease-free survival rate was 67%, and the<br />
overall survival rate was 78%.<br />
Brenyjens et al. used the second CAR T generation, <strong>CD19</strong>-specific <strong>CART</strong> <strong><strong>cell</strong>s</strong> transfected with<br />
CD28/CD3-ζ, to treat 5 patients with relapsed B-ALL. The number and function <strong>of</strong> <strong>CART</strong> <strong><strong>cell</strong>s</strong><br />
were detected by PCR sequenc<strong>in</strong>g. The results showed that <strong>CART</strong> <strong><strong>cell</strong>s</strong> could rapidly produce<br />
anti-tumor effects <strong>in</strong> vivo, and the detection <strong>of</strong> m<strong>in</strong>imal residual disease turned to negative soon<br />
and reached CR. Grupp et al. reported CR <strong>in</strong> 2 patients with refractory and relapsed B-ALL<br />
treated with 2nd generation <strong>CART</strong> <strong><strong>cell</strong>s</strong>. One <strong>of</strong> them was relapsed after umbilical cord blood<br />
allogeneic hematopoietic stem <strong>cell</strong> transplantation and had dual specificity for CD3 and <strong>CD19</strong>.<br />
The monoclonal antibody bl<strong>in</strong>atumomab is resistant. Second-generation <strong>CART</strong> <strong><strong>cell</strong>s</strong> conta<strong>in</strong><strong>in</strong>g 4-<br />
1BB produced by lentiviral vector transfection technique, the number <strong>of</strong> <strong>in</strong>fused T <strong><strong>cell</strong>s</strong> was<br />
(1.4×106-1.2×107)/kg, T <strong>cell</strong> proliferation was more than 1000 times, and peripheral blood was 2<br />
weeks after <strong>in</strong>fusion. Both lymphocytes and neutrophils were significantly elevated. The<br />
lymphocytes were ma<strong>in</strong>ly T <strong><strong>cell</strong>s</strong>, especially <strong>CART</strong> <strong><strong>cell</strong>s</strong>. <strong>CART</strong> <strong><strong>cell</strong>s</strong> can also be present at high<br />
levels <strong>in</strong> the cerebrosp<strong>in</strong>al fluid for at least 6 months, while 2 patients do not have central nervous<br />
system <strong>leukemia</strong>, <strong>in</strong>dicat<strong>in</strong>g the prevention and treatment <strong>of</strong> <strong>CART</strong> <strong><strong>cell</strong>s</strong> for central nervous<br />
system <strong>leukemia</strong>. The ma<strong>in</strong> adverse reactions were agranulocytosis, fever, hypotension, acute<br />
vascular leak syndrome, acute respiratory distress syndrome, elevated ALT and AST, and no acute<br />
toxicity.<br />
At the 56th ASH Conference <strong>in</strong> 2015, Novartis announced the latest cl<strong>in</strong>ical data <strong>of</strong> the <strong>in</strong>dustryrequired<br />
<strong>CART</strong> immunotherapy CTL019. Thirty-n<strong>in</strong>e children with relapsed and refractory ALL<br />
received <strong>CD19</strong>-<strong>CART</strong> <strong>cell</strong> therapy, and 92% (36/39) achieved CR dur<strong>in</strong>g the follow-up period <strong>of</strong><br />
6 months; 69% (27/39) were <strong>in</strong> cont<strong>in</strong>uous remission and the overall survival rate was 74%<br />
(29/39). In a study <strong>of</strong> 24 cases <strong>of</strong> CLL treated with <strong>CD19</strong> CAR T <strong><strong>cell</strong>s</strong>, 50% (12/24) achieved CR;<br />
at a follow-up <strong>of</strong> 9 months, the overall survival rate was 67% (16/24).<br />
At the 2013 ASH meet<strong>in</strong>g, the University <strong>of</strong> Pennsylvania at the United States announced their<br />
latest cl<strong>in</strong>ical research results. 14 patients with relapsed and refractory CLL were treated with<br />
<strong>CART</strong> <strong><strong>cell</strong>s</strong>. The median number <strong>of</strong> <strong>CART</strong> <strong><strong>cell</strong>s</strong> transfused was 1.4×108(0.14×108~5.90×108).<br />
As <strong>of</strong> July 15, 2013, the median follow-up time for all patients was 9.4 (4 to 35) months, and<br />
effective patients were 16 (5 to 35) months. The results showed that 8 out <strong>of</strong> 14 patients were<br />
effectively treated. Among them, 3 patients achieved CR. All patients were followed up for 11, 34,<br />
and 35 months without recurrence. Five patients achieved PR and 2 <strong>of</strong> them appeared after 4<br />
months <strong>of</strong> <strong>in</strong>fusion. relapse.<br />
A phase II study at the University <strong>of</strong> Pennsylvania was reported at the 2013 ASH conference to
f<strong>in</strong>d out the optimal number <strong>of</strong> <strong>CART</strong> <strong><strong>cell</strong>s</strong> for anti-tumor effects. In this study, 10 patients with<br />
relapsed and refractory CLL were enrolled. All patients were treated with <strong>CART</strong> <strong><strong>cell</strong>s</strong>, <strong>of</strong> whom 6<br />
were treated with low amounts (5×107) and 4 patients were treated with high amounts (5×108).<br />
The median follow-up was 3 months. There was no significant difference <strong>in</strong> the efficacy and<br />
adverse reactions between the two groups.<br />
The use <strong>of</strong> <strong>CART</strong> <strong><strong>cell</strong>s</strong> to specifically target <strong>CD19</strong>-positive B-<strong>cell</strong> lymphoma and <strong>leukemia</strong><br />
patients has achieved significant cl<strong>in</strong>ical efficacy and is expected to be widely used. Although the<br />
current <strong>CART</strong> treatment is carried out for a short period <strong>of</strong> time, there is still a lack <strong>of</strong> large-scale<br />
samples, and many adverse reactions and complications need to be gradually discovered and<br />
resolved <strong>in</strong> cl<strong>in</strong>ical trials. However, <strong>in</strong> the cl<strong>in</strong>ical trials reported for the treatment <strong>of</strong> B-<strong>cell</strong><br />
lymphoma and <strong>leukemia</strong>, <strong>CART</strong> showed a very significant effect, especially for relapsed and<br />
refractory patients, thus provid<strong>in</strong>g a basis for a larger-scale, multi-center cl<strong>in</strong>ical study.<br />
Author Bio<br />
As a global company, Creative Biolabs has more than 200 talented and well-tra<strong>in</strong>ed scientists<br />
located <strong>in</strong> different cont<strong>in</strong>ents work<strong>in</strong>g closely with partners from the entire world to develop and<br />
produce medic<strong>in</strong>es <strong>of</strong> tomorrow. Specifically, we are the established lead<strong>in</strong>g expert <strong>in</strong> TCR and<br />
CAR T&NK <strong>cell</strong> immune therapy development, as we <strong>of</strong>fer the one-stop custom services that<br />
cover the entire new drug development pipel<strong>in</strong>e. Additionally, we also <strong>of</strong>fer an exclusive l<strong>in</strong>e <strong>of</strong><br />
ready-to-use TCR T and CAR T&NK <strong>cell</strong> construction products, such as virus packag<strong>in</strong>g,<br />
purification, expansion and titer determ<strong>in</strong>ation kits. Furthermore, we have built up a unique<br />
unparalleled CAR construction and production platform for all four CAR generations.